Human Evolution: Homo habilis to neanderthalensis
A pivotal role for thyroid hormone could best explain the changes from tool users to big-brained, cold-weather hunters in our human history
[Adapted from my 2006 book, Rhythms of Life, with selected references and updated ones]1
The first tool users
Homo habilis retains the title of first hominid tool user. Homo habilis appears to have diverged from a late Australopithecine (Paranthropus boisei, Australopithecus afarensis, or one of their close relations) at the beginning of the Pleistocene, about 2.3 mya.
Fossils of this species are associated with somewhat drier, more open habitats where bovid species like gazelles were plentiful.2
Although these first members of the genus Homo likely scavenged carcasses of large species rather than actively hunting them, to do so meant early Homo habilis had to forage in exposed habitats where carcasses and predators abounded. In other words, they must have had thyroid hormone (TH) rhythms that rendered them tolerant of stresses inherent to much more open habitats than their ancestors experienced because learning to deal with new predators took an abundance of courage.
The scenario for speciation via colonization of a new habitat is the same here as in early domestication: a few stress-tolerant individuals leave the old habitat for a new one, where interbreeding amongst the few thyroid rhythms represented in the group of colonizers very quickly results in novel growth patterns, physical attributes and behaviour.
After only a few generations, the colonizers are different enough in behaviour and reproductive physiology to discourage interbreeding with individuals from the ancestral population. From this point onward, they represent a distinct species that has a unique evolutionary future.
Since the dietary changes faced by early Homo colonizers were not nearly as large as those faced by early Australopithecines, we would not expect the physical changes associated with these speciation events to be as dramatic, and indeed they are not.
Homo habilis was larger than any Australopithecine, with a slightly larger brain.
The slightly different growth and maturation schedules possessed by Homo habilis seem to have affected brain development in such a way as to give them slightly more dexterity (for tool manufacture and use), and perhaps also better decision-making skills. Such neural rearrangements almost certainly gave this species distinct survival advantages in its new exposed habitat.
Although Homo habilis probably still ate some small animals and vegetation, their diet appears to have included a substantial amount of marrow and brains (organs protected by the long-bones and braincase) from scavenged carcasses, obtained by using tools to smash through bone. Such a diet would have been proportionally higher in the essential fatty acids necessary for brain development and function—particularly arachidonic acid (AA) and docosahexaenoic acid (DHA).3
While brains are also a good source of dietary TH, they aren’t as rich in the hormone as liver, kidney or thyroid glands themselves. Unprotected organ tissues are almost always consumed by the primary hunters of any prey and are therefore not usually available to scavengers. Consequently, Homo habilis must have consumed smaller quantities of dietary TH than their Australopithecine ancestors did, even if a modest proportion of their diet still consisted of small whole animals. Selection would thus have favoured individuals whose thyroid glands could produce adequate amounts of TH in the absence of high dietary quantities.
If TH was at times available in less than optimal amounts for early Homo habilis, especially for the brain development of offspring and the mental function of adults, a proportional excess of essential fatty acids such as AA and DHA may have compensated. In other words, an excess of AA and DHA could have optimized what TH was available.
We now know, for example, that DHA in particular is required for the production of transthyretin, the hormone transport molecule that’s particularly important for moving TH around the brain.4
This suggests that abundant fatty acids in the diet of Homo habilis may be of evolutionary significance: when faced with reduced amounts of dietary TH, abundant fatty acids in the diet may have optimized available TH and buffered the selection processes that might otherwise have precipitated more profound and dramatic changes.
Big bodies and big brains on the move—Homo erectus
The next major change in the hominin lineage, especially for physical attributes, is seen in Homo erectus. Erectus had a significantly larger body and brain than its predecessors, with smaller teeth and a more gracile mandible.
Tools and associated animal bones unequivocally establish this species as a hunter of a wide range of medium- and large-sized terrestrial mammals that inhabited open savannah-type environments. Although some of its larger prey species, such as elephants, may still have been scavenged, smaller species were undoubtedly hunted. As primary predators of such prey, Homo erectus would have had ready access to thyroid glands and other unprotected organ tissues would have been readily available.
As a consequence, total dietary TH consumed by Homo erectus would have increased over levels consumed by Homo habilis. Just as for early Australopithecines, individuals that had resilient thyroid rhythms would have retained reproductive function better than others under circumstances of increased consumption of dietary TH.
As TH so strongly controls growth and maturation programs, I suggest that shifts in thyroid rhythms are probably responsible for the morphological changes to brain size and body proportions seen in Homo erectus. The increased brain function and manual dexterity that must have accompanied these developmental timing changes would have conferred distinct survival advantages.5
Once present in the founder population, traits such as larger body size and a more complex brain could rapidly increase in frequency via selection for the growth and maturation programs that underlay them because they were controlled by distinct new thyroid rhythms.
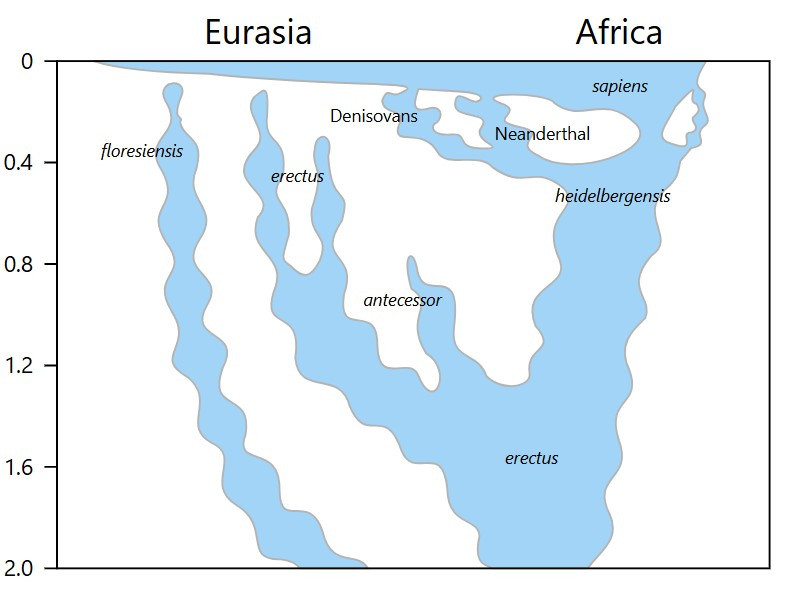
Homo erectus was a successful species that survived for well over one million years. It was also the first hominin to move beyond Africa.
Fossils have been found in both southeast Asia (Indonesia) and western Asia (Georgia) that date to about the same time as these fossil forms are found in Africa (about 1.8 mya).
There is no doubt that Homo erectus lived as successfully in Asian habitats as in African ones. The early presence of Homo erectus in Indonesia, as well as small but distinct skeletal differences and other evidence of a continuous isolated existence in Asia, are the reasons some palaeoanthropologists give for referring to the African form as Homo ergaster and the Asian one as Homo erectus (the Asian fossils, discovered first, retain the original name if these are considered separate species).
In biological terms, however, this situation almost certainly qualifies for the application of subspecies rather than species-level distinctions. Subspecies are used to indicate geographically isolated populations of the same species (which would make the Asian form Homo erectus erectus and the African form Homo erectus ergaster). I therefore refer to both in general discussion as Homo erectus.
A report of fossils with the skeletal distinctions of the Asian form (Homo erectus erectus) in Ethiopia dated at about 1 mya—a form not found in Africa before—seems to confirm this single-species interpretation.6
Nevertheless, what is really critical to understand is that a hominid of essentially identical form that originated in Africa came to live in both Asia and Africa.
That hominid species appears to be the direct and undisputed ancestor of the Homo sapiens lineage.7
Life on the cold front—Homo neanderthalensis
The speciation event that occurred next in the hominid lineage was the emergence of Homo heidelbergensis. A large-bodied, large-brained hominid, this species appears in the fossil record of Africa, Europe and Asia as the first cycles of Pleistocene glaciations kicked into gear.8
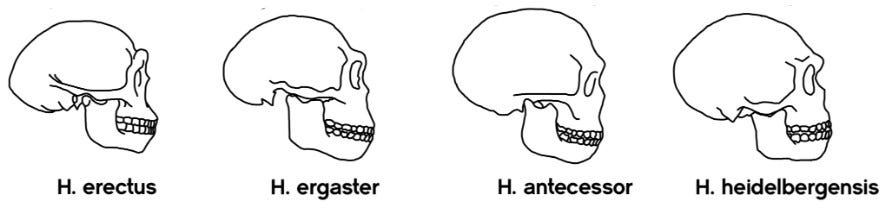
The first species that diverged is called Homo heidelbergensis, as shown in the image below.9
Early Homo heidelbergensis fossils are relatively abundant. They are known from both Africa (Ethiopia at about 600,000 ya; Zambia at about 400,000 ya or so) and Europe (Germany and England at about 320,000–500,000 ya; Spain, France and Hungary at about 250,000–300,000 ya).10
Homo heidelbergensis is considered by many respected palaeoanthropologists to be the common ancestor of Homo neanderthalensis in Eurasia, and Homo sapiens in Africa.
The emergence of Homo heidelbergensis coincides with the onset of cooler and drier Pleistocene environments, a time when many habitats around the world experienced a major turnover of animal communities. Such changes seem to have required these early ancestors to intensify their hunting activities, perhaps in response to increased competition from other carnivorous scavengers for available carcasses. And as we’ve seen, less scavenging and more hunting, means more dietary TH.
Again, an increase in dietary TH would have favoured individuals with the particular thyroid rhythms capable of handling higher levels of ingested TH without disruption of reproductive function. At the same time, much cooler temperatures would have effectively eliminated those with especially cold-sensitive thyroid rhythms from the population, since cold-sensitive animals would likely, at the very least, either fail to breed or be unsuccessful at raising healthy offspring.
As the Pleistocene epoch intensified, further adaptations became necessary in the northern extremes of Homo heidelbergensis territory. Early Neandertals were Homo heidelbergensis colonizers of arctic steppe and tundra.
These habitats expanded and contracted in response to the pronounced glacial and interglacial periods that dominated the climate of Eurasia at this time. Northern colonizers faced even colder temperatures than Homo heidelbergensis had before them. In addition, there were seasonal fluctuations in quantity and variability of prey, both natural consequences of the extreme conditions.
The oldest fossils classified as Homo neanderthalensis date to about 225,000 ya in Germany. The limited geographic area where fossil remains of this species are found suggest that the total range of this species never expanded beyond Europe and western Asia. In other words, Homo neanderthalensis represent successful adaptation of early hominids to a particular set of conditions that were geographically limited and of relatively short duration (in evolutionary terms).
Neandertal ancestors who chose to colonize the harsh and ever-shifting Pleistocene steppe environments must have consisted of a small group (or groups) of individuals who were physiologically tolerant of the relatively severe climatic conditions: they would have possessed one of several naturally-occurring physiological variants that existed naturally within the ancestral Homo heidelbergensis population.
Since TH metabolism is the body’s mechanism for adjusting to cold, individually-unique thyroid rhythms within the ancestral Homo heidelbergensis population would have given some individuals a higher tolerance for the physiological stress of reduced temperatures than others. Temperatures need not have been frigidly arctic, just significantly colder than the habitats of their ancestors. Non-adaptive individuals didn’t necessarily die, they just produced few or no surviving offspring.
The reduced variation of thyroid rhythms present within this small population of early Neandertals would have established a distinct hormonal pattern in their descendants that differed significantly from the common ancestral pattern.
Compounding the selection pressure of cold temperatures would have been the extremely high proportion of the diet necessarily composed of raw meat and organ tissue, simply because far fewer plant products would have been available. Raw meat still appears to have been the dominant dietary component for all hominins at this time, with little compelling evidence for the use of fire for cooking rather than for heat and light.
Homo heidelbergensis in more temperate climates may have consumed moderate quantities of plant material (either year round or seasonally), but it is doubtful that option was available to Neandertals. Neandertals were in many ways simply a more cold-tolerant, more carnivorous form of Homo heidelbergensis but a species distinct from Homo sapiens that came afterward.
I’ll discuss the rise of modern humans in more detail in my next post but for now I’ll note some of the reasons for considering Neandertals and Homo sapiens as distinct species.
Analysis of mineral levels in Homo neanderthalensis bones attest to the fact that a very high proportion of the Neandertal diet was composed of red meat, as could be predicted from the diet of their ancestors and the habitat in which they chose to live, as mentioned above.11
Such a diet would have provided large quantities of dietary TH, especially if thyroid glands and livers were eaten. As a consequence, Neandertals must have had a turn-over rate for TH closer to that seen in carnivorous modern dogs and cats (about 12–13 hours) than the 6.8 days recorded for modern humans. A faster turn-over rate for TH implies that a distinctly different thyroid rhythm must have existed for Neandertals.
And as I’ve argued in previous posts, distinct thyroid rhythm patterns are one way of distinguishing discrete species.
However, more direct evidence that Neandertals possessed distinctive thyroid rhythms comes from new chronological aging techniques that measure incremental growth lines in tooth enamel (perikymata). Analysis of growth lines in the tooth enamel of selected fossils suggests that Homo neanderthalensis had faster postnatal growth rates than do modern humans.12
In addition, analysis of the changes in skull shape that occur during early childhood reveals that Neandertals had faster childhood growth rates relative to modern humans from soon after birth onwards, which indicates Homo neanderthalensis must have possessed a distinct thyroid rhythm.13
Moreover, analysis of Neandertal mtDNA sequences suggests strongly that these hominins were a genetically distinct lineage that had been reproductively isolated for a considerable length of time.14
Did early modern humans and Neandertals interbreed?
However, the close genetic relationship between these species brings up the issue of hybridization: did interbreeding occur when declining numbers of Homo neanderthalensis populations met increasing numbers of early Homo sapiens near the end of the Last Ice Age?
While it appears that hybridization must have happened, the evidence for extensive hybridization claimed by some researchers may be overstated.15
While a few Neandertals (a reconstruction from Wikipedia pictured below) could very easily have been assimilated into populations of early Homo sapiens, it is unlikely such events happened very often. As I believe is the case for recent claims that ancient polar bears interbred extensively with ancient grizzlies throughout their evolutionary history, for these ancient humans it is more likely that genetic results represent evidence of shared common ancestry with Homo heidelbergensis rather than extensive hybridization.16
In other words, shared common ancestry and hybridization are two equally possible interpretations of the same data (which only a few researchers admit) but in most cases, shared common ancestry is the most plausible explanation. However, because hybridization provides a sexier, more news-worthy story, it’s the option most often chosen by authors.
In summary, I contend that dietary changes made necessary by shifts in climate and associated habitat transformations are what drove the speciation changes in human evolution, and that these dietary changes involved varying levels of TH consumed by each species.
In my next essay, I’ll address the rise of early Homo sapiens and discuss how—and why—they became such a successful, globally distributed species.
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria.
Reed, K.E. (1997). Early hominid evolution and ecological change through the African Plio-Pleistocene. Journal of Human Evolution 32, 289-322.
Fleagle, J.G. (1999). Primate Adaptation and Evolution, Second Edition. Academic Press, San Diego.
Horrobin, D. (2001). The Madness of Adam and Eve: How Schizophrenia Shaped Humanity. Bantam Press, London.
Horrobin, D. and Bennett, C.N. (1999). Depression and bipolar disorder: relationships to impaired fatty acid and phospholipids metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, aging and osteoporosis: possible candidate genes. Prostaglandins Leukotines & Essential Fatty Acids 60, 111-167. https://doi.org/10.1054/plef.1999.0037
Kitajka, K., Puskas, L.G., Zvara, A., et al. (2002). The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proceedings of the National Academy of Sciences USA 99, 2619-2624.
McKinney, M.L. (1998). The juvenilized ape myth-our “overdeveloped” brain. BioScience 48, 109-116. https://doi.org/10.2307/1313136
McKinney, M.L. (2002). Brain evolution by stretching the global mitotic clock of development. Human Evolution Through Developmental Change, N. Minugh-Purvis and K. McNamara, K. (eds.), Johns Hopkins University Press, Baltimore, p. 173-188.
Parker, S.T. (2002). Evolutionary relationships between molar eruption and cognitive development in anthropoid apes. Human Evolution Through Developmental Change, N. Minugh-Purvis and K. McNamara, K. (eds.), Johns Hopkins University Press, Baltimore, p. 305-316.
Monson, T.A., Weitz, A.P., Brasil, M.F., and Hlusko, L.J. (2022). Teeth, prenatal growth rates, and the evolution of human-like pregnancy in Homo. Proceedings of the National Academy of Sciences USA119 (41), e2200689119 https://doi.org/10.1073/pnas.220068911
Asfaw, B., Gilbert, W.H., Beyene, Y., et al. (2002). Remains of Homo erectus from Bouri, Middle Awash, Ethiopia. Nature 416, 317-320.
Wood, B. (2020). Birth of Homo erectus. Evolutionary Anthropology 29(6), 293-298. https://doi.org/10.1002/evan.21873
Finlayson, C. 2005. Biogeography and evolution of the genus Homo. TRENDS in Ecology and Evolution 20(8):457-463. https://doi.org/10.1016/j.tree.2005.05.019
Lewis, D. (2022). Ancient skull uncovered in China could be million-year-old Homo erectus. Nature 612, 200-201. https://doi.org/10.1038/d41586-022-04142-0
Stringer, C. (2002). Modern human origins: progress and prospects. Philosophical Transactions of the Royal Society of London B 357, 563-579. https://doi.org/10.1098/rstb.2001.1057
Stringer, C. (2012). What makes a modern human. Nature 485 (7396), 33–35. https://doi.org/10.1038/485033a
Crockford, S.J. (2003). Thyroid rhythm phenotypes and hominid evolution: a new paradigm implicates pulsatile hormone secretion in speciation and adaptation changes. Comparative Biochemistry and Physiology Part A 135, 105–129.
Balter, V., Person, A., Labourdette, N., Drucker, D., Renard, M., et al., 2001. Les Néandertaliens étaient-ils essentiellement carnivores? Résultats préliminaires sur les teneurs en Sr et en Ba de la paléobiocénose mammalienne de Saint-Césaire. Comptes Rendus de l'Academie des Sciences de Paris, Sciences de la Terre et des planétes/Earth and Planetary Sciences 332:59-65.
Ramirez Rozzi, F. (2002). Enamel microstructure in hominids. Human Evolution Through Developmental Change, Minugh-Purvis, N. and McNamara, K. (eds.), Johns Hopkins University Press, Baltimore, p. 319-348.
Minugh-Purvis, N. 2002. Heterochronic change in the neurocranium and the emergence of modern humans. Human Evolution Through Developmental Change, Minugh-Purvis, N. and McNamara, K. (eds.), Johns Hopkins University Press, Baltimore, p. 479-498.
Stringer, C. (2002). Modern human origins: progress and prospects. Philosophical Transactions of the Royal Society of London B 357, 563-579. https://doi.org/10.1098/rstb.2001.1057
Stringer, C. and Crété, L. (2022). Mapping interactions of H. neanderthalensis and Homo sapiens from the fossil and genetic records. PalaeoAnthropology 2, 401–412.
Crockford, S.J. (2023). Polar Bear Evolution: A Model for How New Species Arise. Amazon Digital Services, Victoria. https://www.amazon.com/dp/1778038328
Erikson, A. and Manica, A. (2012). Effect of ancient population structure on the degree of polymorphism shared between modern human populations and ancient hominins. Proceedings of the National Academy of Science 109(35), 13956–13960.
Meneganzin, A. and Bernardi, M. (2023). Were Neanderthals and Homo sapiens ‘good species’? Quaternary Science Reviews 303, 107975.
University of Cambridge (2012). “Research raises doubts about whether modern humans and Neanderthals interbred.” ScienceDaily, 13 August. www.sciencedaily.com/releases/2012/08/120813155521.htm