Speciation: A New Paradigm
A comprehensible model, a few testable hypotheses, and a plausible theory for rapid speciation via thyroid hormone
[This essay has been adapted from a pre-print paper, based on my Ph.D. dissertation and a follow-up book.]1
The critical importance of thyroid hormone
Twenty years ago, I developed a comprehensible model, a few testable hypotheses, and a plausible theory for how new vertebrate species arise rapidly via a non-random process involving a non-genetic driver: thyroid hormone.
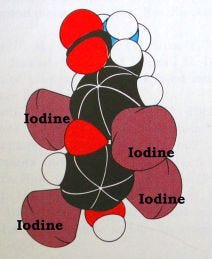
Thyroid hormone is a protein-like molecule with iodine atoms attached (see the figure below). There are several forms with slightly different actions and effects because of the number attached iodine atoms: T4 refers to the form with four atoms of iodine, called thyroxine, while T3 refers to the form with three atoms of iodine. For simplicity, I often use the term ‘thyroid hormone’ to mean whichever form brings about the particular effect I’m discussing, in part because in some cases, it isn’t known which form of thyroid hormone has the most potent effect, only that one or both of them is involved.
Thyroid hormone (TH) is unique in having the same molecular structure across all life forms, which means it can be absorbed directly through the gut from food, as if it were a vitamin.2
Also, the TH needed by a developing embryo for brain and body growth comes initially from its mother — either directly (via the placenta) or indirectly (via TH deposited in egg yolk). As a consequence, the need animals have for TH connects them to the environment and to the generations that came before and after in a way that’s independent of specific gene actions.3
TH has been implicated in heterochronic speciation in a large number of animal species.4 It has been shown to be essential for early embryonic growth and development in virtually all vertebrates and a number of invertebrates.5
Plants and invertebrates have very similar hormones that work in comparable ways but many of them also store the iodine they require in the form of TH, and therefore can use TH for critical functions even though they cannot manufacture it.6
TH not only regulates the function of many individual genes in a time- and dose-dependent manner but it also coordinates growth, development, stress responses, brain function, reproduction, and metabolism (see figure below). This coordination function allows an individual organism to adjust its body functions to routine daily and seasonal changes in its environment, especially light, predation, and temperature.7

In multicellular organisms, TH is the only factor that links the morphological, physiological, reproductive, and behavioural traits known to change in coordinated fashion over evolutionary time. As far as I am aware, this is information that the Modern Synthesis and its population genetic models are simply unable to incorporate.
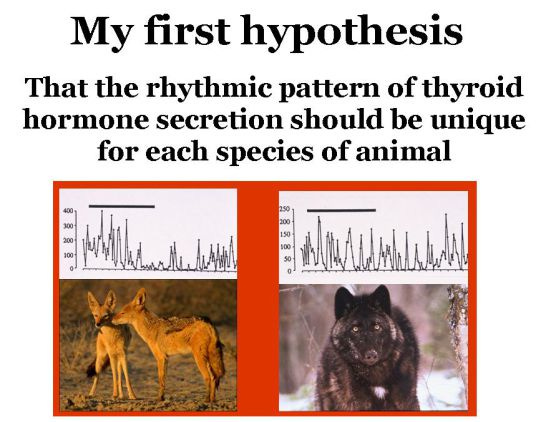
My theory for how most speciation works is based on the premise that each species has a unique rhythm of TH production that varies as the organism grows and ages, which shifts as seasons pass, and controls the species-specific embryonic and post-natal growth of offspring.
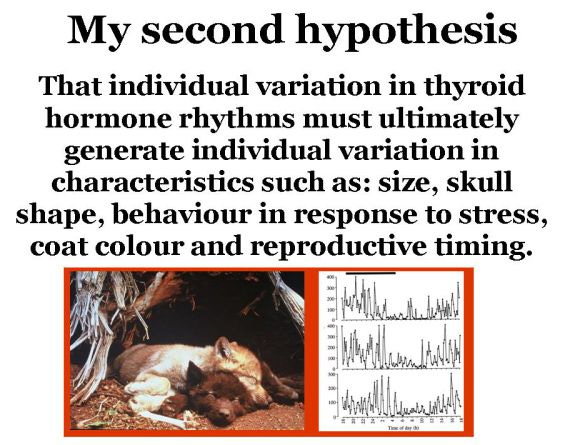
Within each species, this rhythm of TH production also varies slightly from one animal to the next, generating individual TH phenotypes. Individually-unique variants of species-specific TH rhythms thus generate an entire suite of unique physiological, morphological and behavioral characteristics (i.e., individual variation) that is not exclusively genetic in nature.
Furthermore, since TH is critical to embryonic and foetal development from the moment of conception, species- and individual-specific concentrations of TH generated by the maternal system via the placenta, or hormone deposited in egg yolk, provide a generation-to-generation continuity of influence that is similarly non-genetic.
I’m convinced by the available evidence that the biological mechanism that allows species to transform rapidly in response to changing environmental conditions over geological time simply expands in scope the existing hormonal system individual animals are known to use in adapting to daily and seasonal changes during their lifetime.
In many instances, the process revolves around the critical role TH has on behaviour associated with the stress response, including those that make some individuals bold and others timid.8
The concept can be applied to the evolution of all vertebrate and invertebrate lineages from sand dollars to hominids. With slight modification, it also applies to insects and plants that use a slightly different rhythmic hormone to achieve similar results, which they developed because they evolved on land rather than in the ocean.
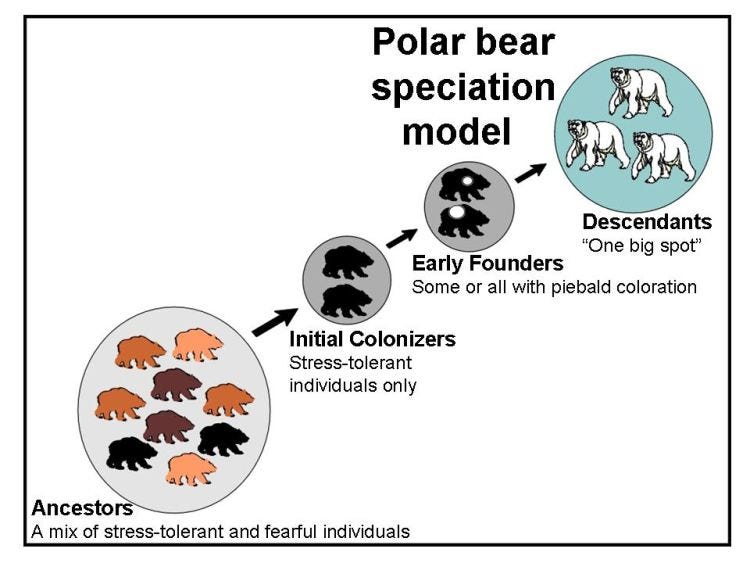
Using polar bears as an example, it works like this:
Given the geochemistry of iodine and its relationship to the evolutionary history of the TH molecule, I suggest this theory also has far-reaching implications for our understanding of the origin of early life forms, and perhaps for life itself.9
Conclusion
Thyroid rhythm theory represents a significant and novel revision of the traditional population genetics-based explanation for speciation and adaptation changes, and has been strengthened by the last twenty years of research.
This paradigm is game-changing for several reasons: 1) it offers a totally new perspective on how changes in phenotype can be achieved with little or no genetic change; 2) it describes a biological mechanism to explain simultaneous changes in a variety of traits, for both speciation and adaptation; 3) it provides a link between individuals, genes, and the environment that exclusively gene-centric explanations lack; 4) it’s testable!
This TH model, perhaps with a bit of tweaking, could form the basis of a 21st century paradigm of evolution that will finally acknowledge the critical importance of heterochrony in speciation.
It is not enough for biologists to acknowledge that heterochrony is a critical evolutionary process if the mathematical models that underpin population genetics cannot incorporate the non-genetic and non-random components that drive it, including the rhythmic production of hormones that respond to environmental cues.10
However, no advances can happen until the powerful people who have built their careers around the Modern Synthesis accept that the way forward will probably originate from a direction — and a person — they never saw coming.
Now that twenty years have passed, I’d like to pursue here on Substack the impact that speciation events, driven by selection for TH phenotypes, have had on human evolution and where that’s left us with respect to TH-dependent health issues.
Stay tuned.
Crockford, S.J. (2023). The species problem and polar bear evolution. ResearchGate preprint, https://doi.org/10.13140/RG.2.2.20218.06089
Crockford, S.J. (2004). Animal Domestication and Vertebrate Speciation: A Paradigm for the Origin of Species. Ph.D. dissertation. University of Victoria, Canada. http://hdl.handle.net/1828/542
Eales, J.G. (1997). Iodine metabolism and thyroid-related functions in organisms lacking thyroid follicles: Are thyroid hormones also vitamins? Proceedings of the Society for Experimental Biology and Medicine 214, 302–317. https://doi.org/10.3181/00379727-214-44098
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria.
Wilson, C.M. and McNabb, F.M.A. (1997). Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. General and Comparative Endocrinology 107, 153–165. https://doi.org/10.1006/gcen.1997.6906
Esposito, A., Ambrosino, L., Piazza, S., et al. (2021). Evolutionary adaption of the thyroid hormone signaling toolkit in chordates. Cells 10, 3391. https://doi.org/10.3390/cells10123391
Hayes, T.B. (1997). Hormonal mechanisms as potential constraints on evolution: examples from the Anura. American Zoologist 37, 482–490. https://doi.org/10.1093/icb/37.6.482
Shkil, F.N. and Smirnov, S.V. (2015). Experimental approach to the hypothesis of heterochronic evolution in lower vertebrates. Paleontological Journal 49, 1624–1634. https://doi.org/10.1134/S0031030115140178
Smirnov, S.V., Kapitanova, D.V., Borisov, V.B., et al. (2012). Lake Tana large barbs diversity: Developmental and hormonal bases. Journal of Ichthyology 52, 861–880. https://doi.org/10.1134/S0032945212110082
Taylor, E. and Heyland, A. (2017). Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Molecular and Cellular Endocrinology 459, 14–20. https://doi.org/10.1016/j.mce.2017.05.019
Bassett, J.H.D. and Williams, G.R. (2003). The molecular actions of thyroid hormone in bone. TRENDS in Endocrinology and Metabolism 14(8), 356–364. https://doi.org/10.1016/S1043-2760(03)00144-9
Chan, S. and Kilby, M.D. (2000). Review: Thyroid hormone and central nervous system development. Journal of Endocrinology 165, 1–8. https://doi.org/10.1677/joe.0.1650001
Chastel, O., Lacroix, A. and Kersten, M. (2003). Pre-breeding energy requirements: Thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. Journal of Avian Biology 34, 298–306. https://doi.org/10.1034/j.1600-048X.2003.02528.x
Gavlik, S., Albina, M. and Specker, J.L. (2002). Metamorphosis in summer flounder: manipulation of thyroid status to synchronize settling behavior, growth, and development. Aquaculture 203, 359–373. https://doi.org/10.1016/S0044-8486(01)00624-X
Hulbert, A.J. (2000). Thyroid hormones and their effects: A new perspective. Biological Review 75, 519–631. https://doi.org/10.1017/S146479310000556X [an excellent summary]
McNabb, A.F.M. and King, D.B. (1993). Thyroid hormone effects on growth, development, and metabolism. In The Endocrinology of Growth, Development, and Metabolism of Vertebrates, Schreibman, M.P., et al. (eds.), p. 393–417. Academic Press, New York.
Salis, P., Roux, N., Huang, D., et al. (2021). Thyroid hormones regulate the formation and environmental plasticity of white bars in clownfishes. Proceedings of the National Academy of Sciences USA 118, e2101634118. https://doi.org/10.1073/pnas.2101634118
Wrutniak, C., Casa, F. and Cabello, G. (2001). Thyroid hormone action in mitochondria. Journal of Molecular Endocrinology 26, 67–77. https://doi.org/10.1677/jme.0.0260067
Zoeller, R.T., Dowling, A.L.S., Herzig, C.T.A., et al. (2002). Thyroid hormone, brain development, and the environment. Environmental Health Perspectives 110(Suppl.3), 355–361. https://doi.org/10.1289/ehp.02110s3355
See Eales, J.G. (1997), above (FN 2).
Farnsworth, E. (2004). Hormones and shifting ecology throughout plant development. Ecology 85, 5–15. https://doi.org/10.1890/02-655
Flatt, T., Moroz, L.L., Tatar, M. and Heyland, A. (2006). Comparing thyroid and insect hormone signalling. Integrative and Comparative Biology 46, 777–794. https://doi.org/10.1093/icb/icl034
See Crockford 2004 and 2006, listed above, for other references, and also
Crockford, S.J. (2009). Evolutionary roots of iodine and thyroid hormones in cell-cell signaling. Integrative and Comparative Biology 49, 155–166. https://doi.org/10.1093/icb/icp053
Crockford, S.J. (2023). Polar Bear Evolution: A Model for How New Species Arise. Amazon KDP, Victoria.
Boissy, A. (1995). Fear and fearfulness in animals. Quarterly Review of Biology 70, 165–191. https://doi.org/10.1086/418981
O’Connor, T.M., O’Halloran, D.J. and Shanahan, F. (2000). The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. Quarterly Journal of Medicine 93, 323–333. https://doi.org/10.1093/qjmed/93.6.323
Crockford, S.J. (2009). Evolutionary roots of iodine and thyroid hormones in cell-cell signaling. Integrative and Comparative Biology 49, 155–166. https://doi.org/10.1093/icb/icp053
Fuge, R. and Johnson, C.C. (2015). Iodine and human health, the role of environmental geochemistry and diet, a review. Applied Geochemistry 63, 282–302. https://doi.org/10.1016/j.apgeochem.2015.09.013
Mourouzis, I., Lavecchia, A.M., and Xinaris, C. (2020). Thyroid hormone signalling: From the dawn of life to the bedside. Journal of Molecular Evolution 88, 88–103. https://doi.org/10.1007/s00239-019-09908-1
Jablonka, E. and Lamb, M. (2020). Inheritance Systems and the Extended Evolutionary Synthesis Elements in the Philosophy of Biology Series, Cambridge University Press, Cambridge.
Laland, K.N., Uller, T., Feldman, M.W., et al. (2015). The extended evolutionary synthesis: Its structure, assumption and predictions. Proceedings of the Royals Society B 282, 20151019. https://doi.org/10.1098/rspb.2015.1019
Pigliucci, M. (2007). Do we need an extended evolutionary synthesis? Evolution 61, 2743–2749. https://doi.org/10.1111/j.1558-5646.2007.00246.x