Speciation via Hormonal Changes to Growth & Development
Rapid speciation involves heterochronic change, which appears to be driven by rhythmic production of thyroid hormone
[This essay has been adapted from a pre-print paper, based on my Ph.D. dissertation and a follow-up book.]1
Speciation and Hormones
One category of information that is missing from the gene-centric Modern Synthesis involves the known effects of rhythmic patterns of hormone production, including well-known circadian rhythms.2
This rhythmic pattern of secretion is a feature of all hormones, which are released at least as often as every few minutes, in bursts that vary in length and intensity.
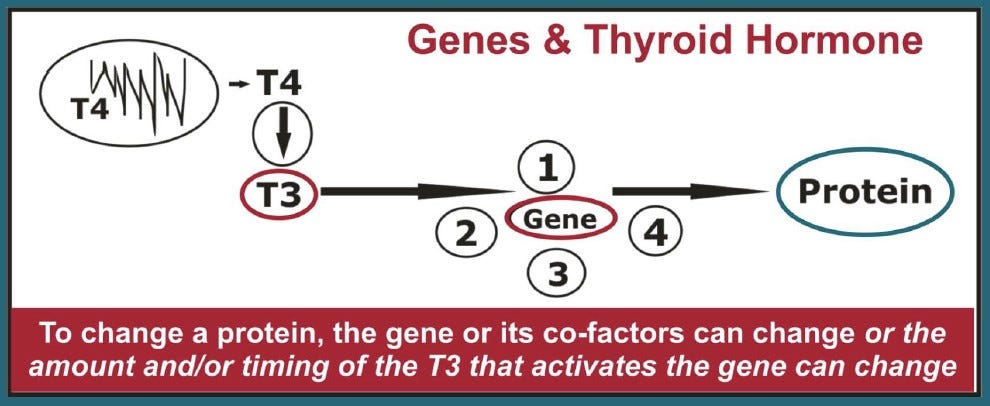
The production of hormones and their feedback mechanisms are now known to have a critical influence not just on how genes work but how the functions of multiple genes are coordinated in plants and animals.3
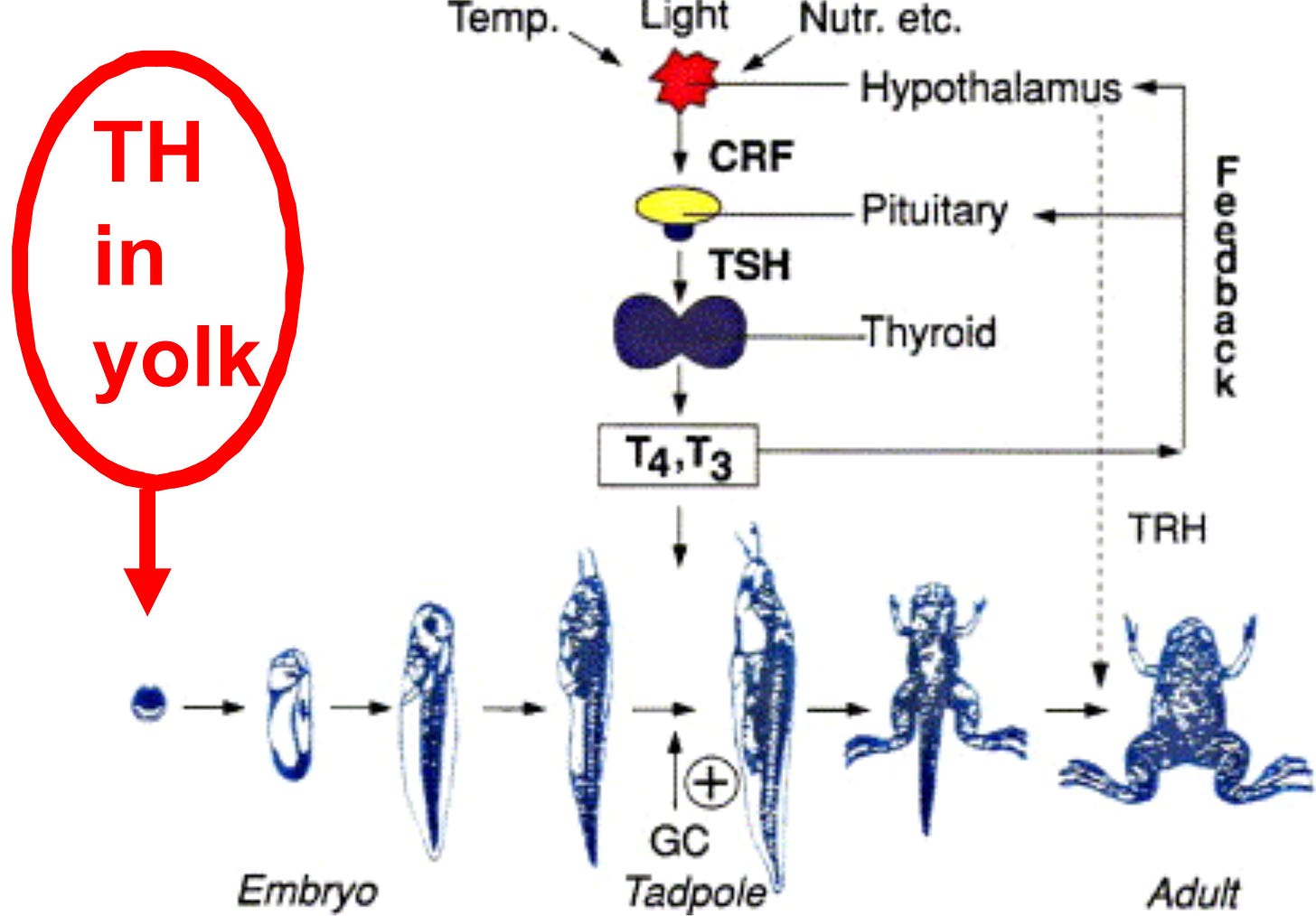
The rhythmic release of hormones — especially thyroid hormones — are known to exert profound effects on the initiation of genetically-controlled life history traits, such as stages of metamorphosis in amphibians, smoltification in salmon, the age of sexual maturity, and the time of year that animals mate or enter hibernation.4
This new awareness of the interplay between hormones and environmental cues has helped us understand, far better than before, how individual animals and populations of animals (i.e., species) adapt in response to environmental change.
Suddenly, the concept of rapid speciation now makes logical sense: organisms have to change almost as fast as environments do. If an organism cannot adapt, there must be a mechanism in place for rapidly generating a new species that better fits the new environmental conditions.
A process known as heterochrony seems to factor heavily into this rapid generation of new species. Heterochrony refers to the kinds of differences between species that result from changes in growth rates or in the timing of critical growth stages, or both, especially during embryonic or postnatal stages of development.
It’s become clear that all phenomena that impact growth and development via heterochrony, including both hormonal and genetic changes, must influence the speed, direction, and magnitude of evolution in response to environmental change.5
Heterochrony and Speciation
Heterochrony is a widespread phenomenon and has been identified in virtually all lineages of multicellular organisms, including vertebrate animals like mammals and fish, invertebrates like insects and clams, and even plants.6
Heterochrony often involves starting and stopping growth stages, changing the rate of foetal or postnatal growth of the ancestral species to various degrees, or all of these — at the same time or one after the other — making possible a wide variety of coordinated shape and size differences in descendant populations, i.e., new species.
In vertebrate animals, heterochrony has been implicated in a number of evolutionary novelties, such as bipedal morphology in human evolution, the reduced hind limbs of whales, the retained juvenile features of modern lungfish, the modified front limbs of bats, and the variation in jaw shapes of fishes, among many others.7
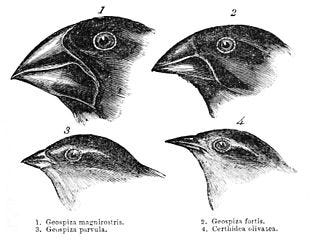
Heterochrony has also been implicated in the small differences that distinguish closely-related species, such as between domestic animals and their wild ancestors, between the chimpanzee and bonobo, and among closely-related species of deer, birds, and fish.8
The Modern Synthesis, however, cannot explain what initiates and controls heterochronic change and ensuing speciation. It has become increasingly apparent that the population genetic models that served well for explaining some cases of speciation and adaptation, including fruit flies and bacteria, are simply not adequate for addressing the phenomenon of heterochrony.
Heterochrony requires a different approach because the interactions between genotypes (i.e., the information encoded in genes) and phenotypes (i.e., the form of the individual organism from the moment an egg is fertilized on forward, including its behavior and hormone physiology), are not linear, one to one relationships but complex webs of interdependence.9
These interactions between genotypes and phenotypes, many of which involve hormone production and their feedback mechanisms, affect all aspects of an organism's shape, behaviour, and life history. While many biologists have acknowledged that this must be true and discussed the problems in depth, so far it has been a theoretical conundrum.10
No one has yet developed a comprehensive evolutionary model for speciation that melds the mathematical tenets of the Modern Synthesis (including the requirement of randomness) with the complex hormonally-driven processes involved in heterochrony. I’m not even sure, at this point, if such a thing is possible.
Twenty years ago I proposed a new paradigm for how new vertebrate species arise rapidly via a non-random process involving a non-genetic driver: thyroid hormone. I’ll lay out this paradigm in my next post.
Crockford, S.J. (2022). The species problem and polar bear evolution. ResearchGate preprint, https://doi.org/10.13140/RG.2.2.20218.06089
Crockford, S.J. (2004). Animal Domestication and Vertebrate Speciation: A Paradigm for the Origin of Species. Ph.D. dissertation. University of Victoria, Canada. http://hdl.handle.net/1828/542
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria.
Reppert, S.M. and Weaver, D.R. (2002). Coordination of circadian timing in mammals. Nature 418, 935–941. https://doi.org/10.1038/nature00965
Farnsworth, E. (2004). Hormones and shifting ecology throughout plant development. Ecology 85, 5–15. https://doi.org/10.1890/02-655
Kohsaka, A. and Bass, J. (2007). A sense of time: How molecular clocks organize metabolism. Trends in Endocrinology and Metabolism 18, 4–11. https://doi.org/10.1016/j.tem.2006.11.005
Miller, A.E.M. and Heyland, A. (2010). Endocrine interactions between plants and animals: Implications of exogenous hormone sources for the evolution of hormone signaling. General and Comparative Endocrinology 166, 455–461. https://doi.org/10.1016/j.ygcen.2009.09.016
Chastel, O., Lacroix, A. and Kersten, M. (2003). Pre-breeding energy requirements: Thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. Journal of Avian Biology 34, 298–306. https://doi.org/10.1034/j.1600-048X.2003.02528.x
Gancedo, B., Alonso-Gomez, A.L., de Pedro, N., et al. (1997). Changes in thyroid hormone concentrations and total contents through ontogeny in three anuran species: evidence for daily cycles. General and Comparative Endocrinology 107, 240–250. https://doi.org/10.1006/gcen.1997.6922
Hulbert, A.J. (2000). Thyroid hormones and their effects: A new perspective. Biological Review 75, 519–631. https://doi.org/10.1017/S146479310000556X
Shi, Z.H. and Barrel, B.K. (1992). Requirement of thyroid function for the expression of seasonal reproductive and related changes in the red deer (Cervus elaphus) stags. Journal of Reproduction and Fertility 94, 251–260. https://doi.org/10.1530/jrf.0.0940251
Tomasi, T.E. and Mitchell, D.A. (1994). Seasonal shifts in thyroid function in the cotton rat (Sigmodon hispidus). Journal of Mammalogy 75, 520–528. https://doi.org/10.2307/1382576
Gould, S.J. (2002). The Structure of Evolutionary Theory. Belknap Press, Cambridge.
McNamara, K.J. and McKinney, M.L. (2005). Heterochrony, disparity, and macroevolution. Paleobiology 31 (Supple.), 17–26. https://doi.org/10.1666/0094-8373(2005)031[0017:HDAM]2.0.CO;2
West-Eberhard, M.J. (2003). Developmental Plasticity and Evolution. Oxford University Press, Oxford.
Li, P. and Johnston, M.O. (2000). Heterochrony in plant evolutionary studies through the twentieth century. Botanical Reviews 66, 57088. https://doi.org/10.1007/BF02857782
Berge, C. (2002). Peramorphic processes in the evolution of the hominid pelvis and femur. In Human Evolution Through Developmental Change, Minugh-Purvis, N. and McNamara, K. (eds.), p. 381–404. Johns Hopkins University Press, Baltimore.
Thewissen, J.G.M. (1998). The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Plenum Press, New York.
Joss, J.M.P. (2006). Lungfish evolution and development. General and Comparative Endocrinology 148, 285–289. https://doi.org/10.1016/j.ygcen.2005.10.010
Sears, K. E., Behringer, R.R., Rasweiler IV, J.J. and Niswander, L.A. (2006). Development of bat flight: Morphologic and molecular evolution of bat wing digits. Proceedings of the National Academy of Sciences USA 103, 6581–6586. https://doi.org/10.1073/pnas.0509716103
Svensson, M.E. and Haas, A. (2005). Evolutionary innovation in the vertebrate jaw: A derived morphology in anuran tadpoles and its possible developmental origin. BioEssays 27, 526–532. https://doi.org/10.1002/bies.20224
Crockford, S.J. (2004). Animal Domestication and Vertebrate Speciation: A Paradigm for the Origin of Species. Ph.D. dissertation. University of Victoria, Canada. http://hdl.handle.net/1828/542
Gross, M.R. (1998). One species with two biologies: Atlantic salmon (Salmo salar) in the wild and in aquaculture. Canadian Journal of Fisheries and Aquatic Science 55 (suppl.), 131–144. https://doi.org/10.1139/d98-024
Milla, S., Pasquet, A., Mohajer, L.E., and Fontaine, P. (2021). How domestication alters fish phenotypes. Reviews in Aquaculture 13(1), 388–405. https://doi.org/10.1111/raq.12480
Beringer, V. Deschner, T., Murtagh, R. et al. (2014). Age-related changes in thyroid hormone levels of bonobos and chimpanzees indicate heterochrony in development. Journal of Human Evolution 66, 83–88. https://doi.org/10.1016/j.jhevol.2013.09.008
Geist, V. (1998). Deer of the World: Their Evolution, Behavior, and Ecology. Stackpole Books, Mechanicsburg, PA.
Grant, P.R. and Grant. B.R. (2002). Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296, 707–711. doi:10.1126/science.1070315
Grant, P.R., Grant, B.R. and Abzhanov, A. (2006). A developing paradigm for the development of bird beaks. Biological Journal of the Linnaean Society 88, 17–22. https://doi.org/10.1111/j.1095-8312.2006.00595.x
Shkil, F.N. and Smirnov, S.V. (2015). Experimental approach to the hypothesis of heterochronic evolution in lower vertebrates. Paleontological Journal 49, 1624–1634. https://doi.org/10.1134/S0031030115140178
Smirnov, S.V., Kapitanova, D.V., Borisov, V.B., et al. (2012). Lake Tana large barbs diversity: Developmental and hormonal bases. Journal of Ichthyology 52, 861–880. https://doi.org/10.1134/S0032945212110082
Woronowicz, K.C., Esin, E.V., Markevich, G.N., et al. (2023). Phylogenomic analysis of the Lake Kronotskoe species flock of Dolly Varden charr reveals genetic signatures of sympatric radiation. bioRxiv 2023.02.24.529919. https://doi.org/10.1101/2023.02.24.529919
West-Eberhard, M.J. (2003). Developmental Plasticity and Evolution. Oxford University Press, Oxford.
Laland, K.N., Uller, T., Feldman, M.W., et al. (2015). The extended evolutionary synthesis: Its structure, assumption and predictions. Proceedings of the Royals Society B 282, 20151019. https://doi.org/10.1098/rspb.2015.1019