Thyroid hormones and fetal growth
TH has indirect effects, by varying the way that genes function, but also through direct actions on cells and tissues – especially in the production of steroid hormones (including sex hormones).
[Adapted from my 2006 book, Rhythms of Life, with selected references]1
As discussed previously in relation to the phenomenon of piebaldness (sometimes called whitespotting), thyroid hormone (TH) is required for normal embryonic growth at all stages—probably from conception onward.
The TH required is supplied by the maternal system either directly (for mammals, via the placenta) or via reserves stored in egg yolk (for non-placental animals like birds, reptiles and amphibians). Many of the myriad small steps that make up embryonic growth, particularly for brain tissue, are known to be controlled by TH.2
For example, if TH is not supplied in an appropriate time- and dose-dependent manner during embryonic growth, the migration and maturation of emerging brain cells is altered, resulting in permanently impaired brain function (both too much and too little can have equally devastating effects). Experiments on newborn rats suggests that TH also controls the absolute size of the brain because disruption can diminish the development of certain cell types. The hearing apparatus is particularly sensitive to TH, both before and after birth.3
But how does TH do all these things? TH has indirect effects, by varying the way that genes function, but also through direct actions on cells and tissues – especially in the production of steroid hormones (including sex hormones). Some general details are given below; even more detail, for those who want it, are given at the end of the essay.
TH and other hormones
Direct and indirect (gene-promoting) actions of thyroid hormone are not the only way that thyroid hormone effects growth and development: remember that thyroid hormone is also required for the production and actions of other hormones.
Growth and sex hormones, for example, are both required for growth of the skeleton and both are dependent on thyroid hormone for their release from the pituitary as well as for their actions on bone.4
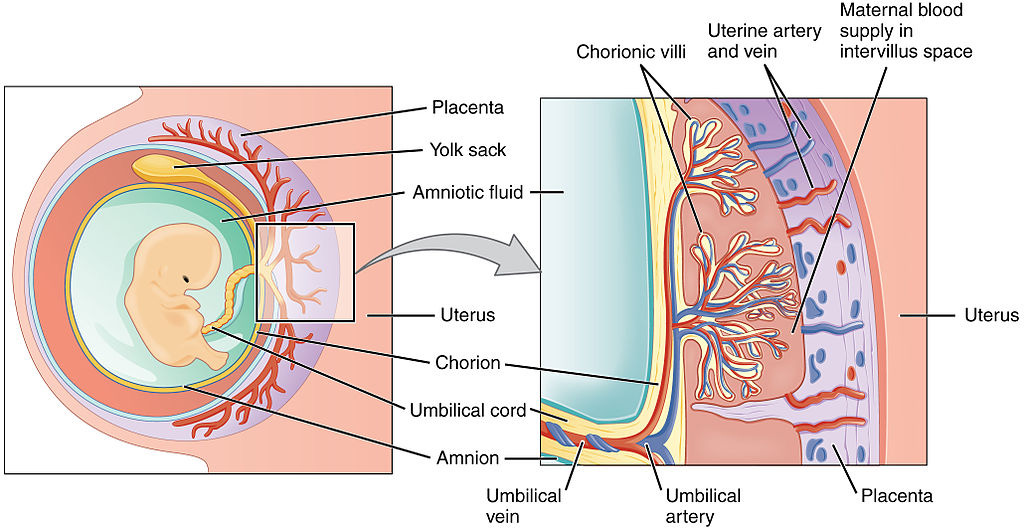
TH in egg yolk and placenta
For animals that develop internally, all required hormones (as well as vitamins, essential fatty acids and growth factors) are supplied by the mother, via the placenta. The placenta itself may be dependent on T3 for its formation during early pregnancy, and later is capable of producing several hormones required by the fetus that can’t pass through from the maternal system (such as growth hormone).
For animal embryos that develop externally (inside an egg), all required hormones and other essential nutrients are deposited in the egg yolk by the mother.5
The exact amounts of hormone incorporated are controlled by the maternal system, as shown by colleagues Morgan Wilson and Anne McNabb, who found that the T4 concentration of egg yolk varied with the thyroid hormone status of the hen in Japanese quail.6
This process puts non-mammalian vertebrates—such as birds, reptiles, amphibians and fish—in a similar position as placental mammals in regards to the mother maintaining control over early embryonic growth of her offspring. Marsupials such as kangaroos, which spend part of their embryonic growth period outside their mother’s womb but inside her pouch firmly attached to a teat, get the thyroid hormone they require for late fetal development from her milk.7
Without getting into too much detail, T3 has been shown to be critical during embryonic, neonatal and adult muscle fibre formation, and as already discussed, both T4 and T3 have been identified as essential, in animal models such as the rat, for various critical stages in the development of the central nervous system.8
Maternal T3 stimulates the production of epidermal growth factor (EGF) as well as oestradiol (via effects on steroidogenesis) in the placenta. In fact, maternal T3 may play a key role in the development of the placenta itself.9
In addition, all known thyroid hormone receptors (TRs) are expressed by the placenta, which manufactures a number of other substances critical to development, including steroid hormones (such as progesterone, estrogen, testosterone) and placental lactogen (a growth factor related to GH required by the fetus).
The demonstrated role of thyroid hormone in embryonic growth provides a really significant insight into how control over species-specific growth can be achieved.
Because of the critical role played by maternal thyroid hormone in early embryonic development (both directly and indirectly), it’s clear that the precise endocrine physiology possessed by a mother (or passed along by her into her eggs or milk) must control the early development of her offspring—and continues to influence growth until they are born.10
Only from that point during development when the fetal thyroid gland becomes functional (which varies from species to species) do genes controlling thyroid hormone function that were contributed by the father have an opportunity to be expressed in their offspring.
The distinct stages at which offspring growth is affected by each set of parental genes controlling thyroid hormone function may provide a partial explanation for why the physical form of offspring from hybrid crosses differs depending on which species is the dam.
For example, consider the hybrid offspring from horses and donkeys: given the same breeds or types of parents in both cases, when the female is a horse and the male a donkey, the offspring is called a mule because it has certain consistent physical characteristics (including larger size) that distinguish it from a hinny, the offspring that results when the female is a donkey and the male a horse.
A number of similar examples are known among wild animals of all kinds, including mammals, fish and amphibians.
It’s become patently clear that we know precious little about developmental timing and growth rates in different species of animals. Given the importance of changing rates of growth and timing of sexual maturation to evolution, we are in rather desperate need of more research in this area.
But with that said, we have to remember the two factors that are essential for evolution to occur: individual variation and changes to the environment.
Changing environments often mean changing food supplies. I’ll discuss this connection between thyroid hormone and food supply in the next round of posts as I delve into the topic of human evolution, with the ultimate objective of discussing the implication of our evolutionary history on modern human health.
Expert-level details of thyroid hormone action
[for future reference and for those who want some nitty-gritty details]
Based on experimental studies, embryonic neural crest tissue has been shown to be the source of several essential cell lineages that are dependent on retinoid acid and T4 for properly timed migration, proliferation and maturation, including epidermal and choroidal pigment cells, neurons and glia of the peripheral nervous system, neuroendocrine and inner ear sensory cells, and pharyngeal arch-derived tissues of the face and neck. In the developing digestive system, thyroid hormones are known to be responsible for the differentiation of the epithelial lining of the small intestine (where nutrient absorption occurs) and to affect the timing of tooth eruption and tooth enamel formation.11
As a gene regulator, T3 has been found to bind to both nuclear and mitochondrial thyroid hormone receptors to form a ligand-receptor complex. This T3-receptor complex then binds to a specific DNA sequence located within the promoter region (the thyroid responsive element, or TRE) of a number of genes, triggering the transcription of gene products (enzymes and proteins) within cell nuclei and mitochondria.12
Thus, TH in its T3 state, has been found to influence the transcription of a wide variety of genes, including those involved in the synthesis of lung surfactants, nerve and epidermal growth factors, and a large number of critical brain function proteins.13
Direct (non-genomic) effects of T3 have also been demonstrated; that is, some interactions do not involve the binding of the hormone to a receptor or TRE. In particular, these non-genomic effects have been identified in cell and mitochondrial membranes and have been shown to control several essential functions.
For example, T3 has been found to stimulate Ca2+-ATPase production in cell membranes, to increase oxidative phosphorylation in mitochondria and to induce the synthesis of Na+/K+-ATPase needed to activate the so-called “sodium pump” that produces heat in mitochondria.14
Of particular importance to this discussion is an essential role for T3 in steroidogenesis (the synthesis of steroid hormones from a cholesterol substrate, which takes place in mitochondrial inner membranes and is required for manufacture of all glucocorticoids, catecolamines, testosterone, and estrogen).
T3 has been shown to stimulate steroidogenic acute regulatory protein (StAR) gene expression in a time- and dose-dependent manner (increasing production of the enzyme needed to convert cholesterol into pregenolone, the first step in steroid hormone manufacture) in Leydig cells of testes of male rats thus significantly increasing testosterone production.15
Although this has yet to be proven a general phenomenon affecting all steroidogenic cells, T3 has been shown to regulate StAR protein-mediated steroidogenesis in adrenal cortex tissue and to induce gonadal growth in Japanese quail.16
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria.
Jones, S.A., Thoemke, K.R. and Anderson, G.W. (2005). The role of thyroid hormone in fetal and neonatal brain development. Current Opinion in Endocrinology and Diabetes 12, 10-16.
Yen, P.M. (2001). Physiological and molecular basis of thyroid hormone action. Physiological Reviews 81(3), 1097-1142.
Yen, P.M. (2003). Molecular basis of resistance to thyroid hormone. TRENDS in Endocrinology and Metabolism 14(7), 327-333.
Keep in mind that embryonic growth in rats and mice—the animals used most often as laboratory proxies for scientific studies of human fetal development—is quite different from that in humans: rats and mice are about 12–14 days old before their body and brain growth is equivalent to that of humans at birth.
This means that the growth of rats and mice in the two weeks after birth is equivalent to human fetal growth during the last three months of gestation. Scientists can easily vary conditions associated with growth in newborn rats and mice experimentally to mimic affects on human infants during the final stages of pregnancy. These differences are of course taken into account when designing experiments with rodents and when interpreting what the results mean in human growth terms.
Anderson, G.W., Schoonover, C.M. and Jones, S.A. (2003). Control of thyroid hormone action in the developing rat brain. Thyroid 13(11), 1039-1056.
Chan, S. and Kilby, M.D. (2000). Review: Thyroid hormone and central nervous system development. Journal of Endocrinology 165, 1-8.
Bassett, J.H.D. and Williams, G.R. (2003). The molecular actions of thyroid hormone in bone. TRENDS in Endocrinology and Metabolism 14(8), 356-364.
De Pablo, F. (1993). Introduction, The Endocrinology of Growth, Development, and Metabolism of Vertebrates, Schreibman, M.P., Scanes, C.G. and P.K.T. Pang (eds.), Academic Press, New York, p. 1-11.
Elinson, R.P. (1987). Change in developmental patterns: embryos of amphibians with large eggs. Development as an Evolutionary Process, R.A. Raff and E.C. Raff (eds.), A.R. Liss Inc., New York, p. 1-21.
Liu, Y-W. and Chan, W-K. 2002. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation 70(1):1432-1436.
O’Steen, S. and Janzen, F.J. 1999. Embryonic temperature effects metabolic compensation and thyroid hormones in hatchling snapping turtles. Physiology, Biochemistry and Zoology 72(5):520-533.
Rol'nik, V.V. 1970. Bird Embryology. Israel Program for Scientific Translations, Jerusalem.
Wilson, C.M. and McNabb, F.M.A. (1997). Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. General and Comparative Endocrinology 107, 153-165.
Richardson, S.J., Monk, J.A., Shepherdly, C.A., et al. (2005). Developmentally regulated thyroid hormone distributor proteins in marsupials, a reptile, and fish. American Journal of Physiology: Regulatory and Integrative Comparative Physiology 288, R1264-R1272.
Dubois-Dalcq., M. and Murray, K. (2000). Why are growth factors important in oligodendrocyte physiology? Pathological Biology 48, 80-86.
Gardahaut, M.F., Fontaine-Perus, J., Rouaud, T., et al. (1992). Developmental modulation of myosin expression by thyroid hormones in avian skeletal muscle. Development 115, 1121-1131.
Lavado-Autric, R., Ausó, E., Garcia-Velasco, J.V., et al. (2003). Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. Journal of Clinical Investigation 111, 1073-1082.
Smallridge, R.C. and Ladenson, P.W. (2001). Hypothyroidism in pregnancy: consequences to neonatal health. Journal of Clinical Endocrinology and Metabolism 86, 2349-2353.
Kilby, M.D., Verhaeg, J., Gittoes, N., et al. (1998). Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR). Journal of Clinical Endocrinology & Metabolism 83(8), 2964-2971.
Burrow, G.N. (1997). Editorial: mothers are important! Endocrinology 138, 3-4.
Piosik, P.A., van Groenigen, M., van Doorn, J., et al. (1997). Effects of maternal thryoid status on thyroid hormones and growth in congenitally hypothyroid goat fetuses during the second half of gestation. Endocrinology 138, 5-11.
Barres, B.A., Lazar, M.A. and Raff, M.C. (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097-1108.
Chan, S. and Kilby, M.D. (2000). Review: Thyroid hormone and central nervous system development. Journal of Endocrinology 165, 1-8.
Noren, J.G. and Alm, J. 1983. Congenital hypothyroidism and changes in the enamel of deciduous teeth. Acta Paediatrica Scandinavica 72(4), 485-489.
Pirinen, S. (1995). Endocrine regulation of craniofacial growth. Acta Odontologia Scandinavica 53(3), 179-185.
Chan, S. and Rovet, J. (2003). Thyroid hormones in fetal central nervous system development. Fetal and Maternal Medicine Review 14, 177-208.
Garcia-Segura, L.M. and McCarthy, M.M. (2004). Role of glia in neuroendocrine function. Endocrinology 145(3), 1082-1086.
Kőhrle, J. (2000). Thyroid hormone metabolism and action in the brain and pituitary. Acta Medica Austriaca. 27(1), 1-7.
Oppenheimer, J.H. and Schwartz, H.L. (1997). Molecular basis of thyroid hormone dependent brain development. Endocrine Review 18, 462-475.
Davis, P.J., Davis, F.B. and Lawrence, W.D. (1989). Thyroid hormone regulation of membrane Ca2(+)-ATPase activity. Endocrine Research 15(4), 651-682.
Hadley, M.E. (2000). Endocrinology, Fifth Edition. Prentice-Hall Inc, New Jersey.
Hulbert, A.J. (2000). Thyroid hormones and their effects: A new perspective. Biological Review 75, 519–631. https://doi.org/10.1017/S146479310000556X
Shin, D-J. and Osborne, T.F. (2003). Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2). Journal of Biological Chemistry 278(36), 34114-34118.
Wrutniak, C., Casa, F. and Cabello, G. 2001. Thyroid hormone action in mitochondria. Journal of Molecular Endocrinology 26, 67-77.
Manna, P.R., Tena-Sempere, M. and Huhtaneimi, I.T. (1999). Molecular mechanisms of thyroid hormone-stimulated steroidogenesis in mouse Leydig tumor cells. Journal of Biological Chemistry 272(9), 5909-5918.
Jannini, E.A., Ulisse, S. and D’Armiento, M. 1995. Thyroid hormone and male gonadal function. Endocrine Reviews 16(4), 443-459.
Jefcoate, C. (2002). High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. Journal of Clinical Investigation 110(7), 881-890.
Yoshimura, T., Yasuo, S., Watanabe, M., et al. (2003). Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426, 178-181.