Hypothyroidism: Our Evolutionary Legacy
Depression, heart failure, infertility, weight gain, and other symptoms of thyroid dysfunction are a unique health consequence of our evolutionary history
[Adapted from my 2006 book, Rhythms of Life, with selected references, including some updated ones]1
When applied to human evolution, the concept that selection for particular variants of thyroid hormone (TH) metabolism over time drives rapid evolutionary change in response to environmental and dietary shifts, explains some uniquely-human health issues. Thyroid dysfunction—viewed as a legacy of our evolutionary history—makes evolution personal.
Modern humans, with their very slow turnover rate for T4, appear to be well adapted to a diet lacking any appreciable amounts of TH.2
However, our tolerance to fluctuations in TH levels is very low indeed, and either too much (hyperthyroidism) or too little (hypothyroidism) makes us decidedly ill. Hypothyroidism is by far the most common imbalance: having too much thyroid hormone is comparatively rare.3
Although we don’t have data from 10,000 years ago to know if the prevalence of hypothyroidism is a new phenomenon for humans, we do know there are millions of people worldwide currently afflicted by its symptoms, with a recent estimate suggesting this might comprise as much as 10% of the entire population.4
In other words, current rates of hypothyroidism now rival diabetes rates worldwide.
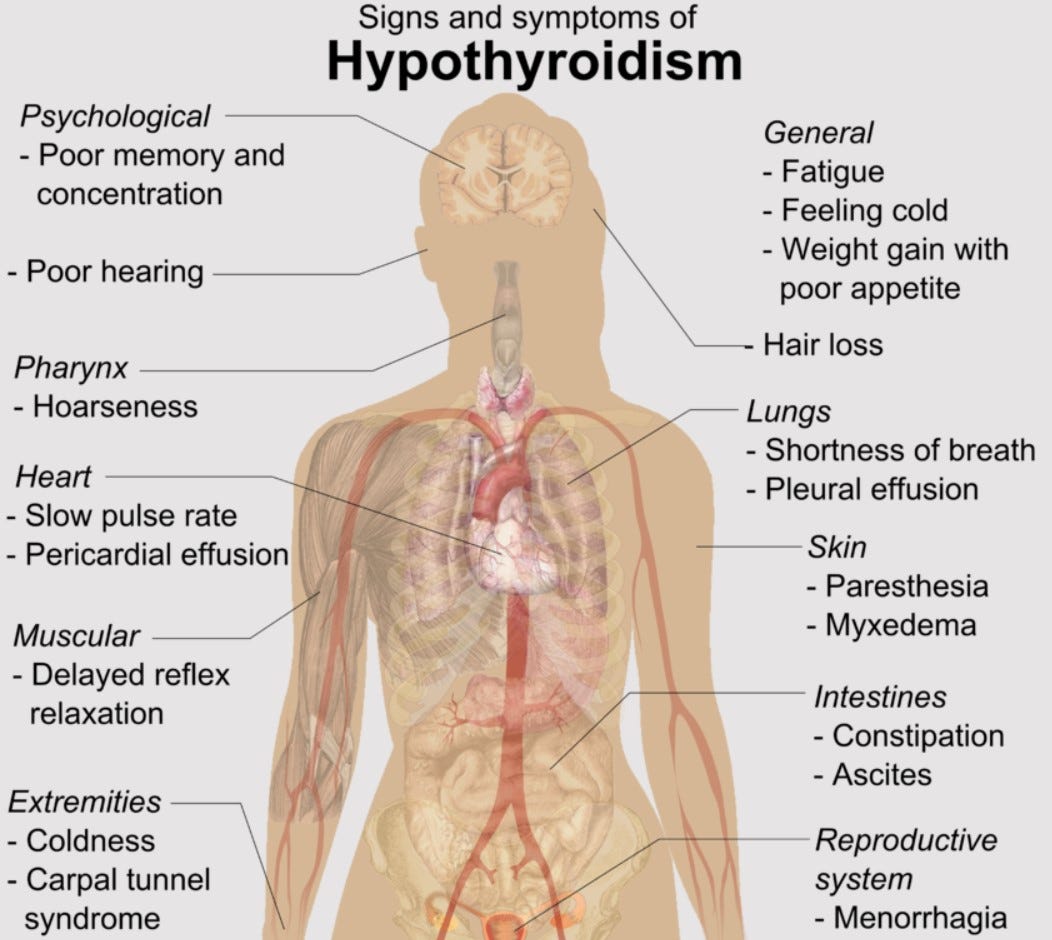
The list of symptoms is long and diverse—not surprisingly, considering how many functions depend on TH—and can be present in often idiosyncratic fashion, with some (but not necessarily all of those listed below) occurring together, include:
1) Brain function: depression; poor memory; inability to concentrate; mood swings; sleep disturbances; anxiety
2) Skin/hair quality: dry skin; puffy eyes; hair loss; coarse or brittle hair
3) Reproductive functions: irregular menstruation; heavy menstruation; infertility; miscarriage; birth defects
4) Circulation/respiration functions: high cholesterol; high blood pressure; congestive heart failure; sleep-associated breathing problems
5) Muscle/joint function: muscle cramps; muscle weakness; muscle and joint pain; deep voice; hoarse voice; “pins and needles” in hands/feet
6) Metabolic functions: heat/cold intolerance; weight gain; constipation; general tiredness
Hypothyroidism has also been strongly linked to hyperactivity disorders in children, to postpartum depression, schizophrenia, and to some kinds of post-traumatic stress disorders.5
People do not often die of these disorders but they are very often rendered under-productive or even non-productive.6
As alluded to above, we don’t have firm estimates of how many people suffer from imbalances because we don’t really know the precise rate of under-diagnosis using traditional methods of analysis.
For example, more than 20 years ago the comprehensive Hollowell study tested over 17,000 Americans for serum TSH, T4 and thyroid antibody levels. Extrapolation of their survey results predicted that within the United States alone more than eight million people would probably test positive for hypothyroidism and an additional 700,000 people unknowingly have hyperthyroidism.7
In addition, the survey suggested that a significant number of people (more than 30%) who were already taking thyroid supplements for hypothyroidism were not getting enough. Another study on an even larger population of almost 26,000 participants got similar results.8
As high as these numbers seem, they are probably still far below the real figures because both studies used a fairly wide range of serum TSH concentration readings (0.5 to 5.0mlU/liter) as indicators of normal thyroid function (values exceeding high end values for “normal” TSH—in this case, over 5.0—indicate hypothyroidism).
TSH is the hormone released by the pituitary gland in response to hormonal stimulation by the hypothalamus, when TH levels in the blood fall or rise. In principle, a small drop in TH concentration should generate a very large rise in TSH values (about 100X more, which is one of the reasons TSH, rather than T4, was chosen as an indicator of thyroid function—all other hormones are measured directly.9
In the late 1990s and early 2000s, there was a push by some researchers and clinicians to recommend narrowing the range indicating normal TSH function down to 0.3–3.04mlU/liter or even lower, in part because about 95% of normal individuals actually have values below 2.5mlU/liter but also because the precision of the TSH test itself had improved since it was developed in 1976.10
However, that battle appears to have been lost or abandoned. Currently, most official reference ranges usually max out at 4.0-4.5mlU/liter. It’s unclear to me whether clinicians promoting a narrower range were shut down through “consensus statements” by powerful organizations or whether there has been a genuine shift towards agreement that the slightly larger range really does give better results.11
In contrast, it appears that early evidence of individually distinct TSH ranges have gained traction to the point of recommendations that this information be used for clinical evaluation—to treat patients as individuals, rather than as members of a population.12
For example, a recent Danish study on sixteen healthy males tested thyroid function repeatedly over one year and found a wide range of variation between individuals for all values (serum T4, T3, free T4 and TSH) but a very narrow range of variation within them. In other words, each of these men felt well at one particular level of thyroid function that was fairly consistent for him over the space of a year—the test values were not bouncing all over the place from one month to the next but were individually stable.13
Similarly, a later study that compared thyroid function values (free T3 and T4, and serum TSH) among and between sets of identical and non-identical twins generated results that prompted the authors to report that “each individual may have a genetically determined thyroid function set-point” [interestingly, the Hollowell study mentioned above (among others) found non-Hispanic blacks consistently show a much lower incidence of hypothyroidism, suggesting they have a more resilient thyroid function than Caucasians or Hispanics).14
Although neither of these experiments were measuring hormone rhythms (testing many times per hour), the results are nevertheless consistent with my contention that TH function should show distinct individual variation.
However, along with the issue of individual variation in thyroid function, there is also a potential problem with the reliance on the TSH test as the primary diagnostic tool for determining when TH levels have gone awry. Its use is defended on the grounds that TSH and TH levels in the blood are intimately tied. On the other hand, there is a considerable lag effect between changing TH levels and TSH readings: “at least 6 weeks is needed before retesting TSH following a change in dose of synthetic thyroid hormone.”15
This is why replacement doses for patients with hypothyroidism are usually given in 25 microgram increments every 6–8 weeks, with retesting for TSH at those intervals (usually until the level falls below the upper threshold the doctor is using).
But that 6–8 week time lag suggests that TSH isn’t really an immediate indicator of TH status, and may not reflect symptoms that patients are experiencing.
In addition, as I’ve discussed before, the thyroid gland is known to have a direct nerve connection (via sympathetic nerves) to the hypothalamus and the rhythm control centre of the hypothalamus (the suprachiasmatic nucleus, SCN), which means that thyroid hormone secretion can be stimulated or depressed independently of changing TSH levels. In fact, it appears that the response of the thyroid gland to TSH varies depending on the activity of these nerves.16
Altogether, mounting evidence suggests that steadfast reliance on the TSH test as the best indicator of optimal thyroid function may be causing physicians undue problems in diagnosis and treatment: it may be the best we have now, but may not be the optimal test we could have if individual variation and hormone rhythms were taken into account.
The same may be true in veterinary medicine, where the TSH test is also the primary diagnostic tool for measuring hypothyroidism in animals, and where its use has been similarly disputed.17
In dogs, the normal range for TSH concentrations established by laboratories came from testing a few dogs from many breeds to generate a general “dog” value. It’s now acknowledged that each dog breed probably has a different range of values for “normal.” However, the possibility that there might be individual variation in values for normal thyroid function are not taken into account here either.18
Most importantly, the rhythmic nature of both TH and TSH secretion is still rarely acknowledged in either human or veterinary clinical medicine. Most significantly, experiments on several hormones have shown that when these rhythms are disrupted, clinical symptoms of malfunction appear.19
However, a similar approach in terms of analyzing daily thyroid rhythms has largely not been taken in the study of thyroid disorders.
As a consequence, for both human and canine cases, when single-sample lab tests measured against a population-based range of “normal” are used as a guide, the diagnosis of hypothyroidism is haphazard and the treatment prescribed is often ineffective in resolving all symptoms in individual patients.
I contend this is because we simply aren’t recognizing the individual variation in TH function that exists or the disruption to thyroid rhythms that may underlie individual symptoms that have their roots in how we came to be human.
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria. [Note: I had initially included a chapter in my 2004 Ph.D. dissertation on the implications of my TH speciation theory for human health but was asked by my committee to remove it. They felt it was so interesting and compelling that it distracted from the primary goal of the thesis, which was to describe the speciation theory.]
Turnover rate is the time taken for a measured amount of TH (in its T4 form) to become reduced to half its original value (called its half-life), which reflects differences in TH metabolism after thyroid hormone is released. The average half-life of T4 is 13 hours in dogs, 16.6 hours in cats, and 6.8 days in humans—in other words, carnivores like dogs and cats can (or must) replenish T4 levels much more often than omnivorous humans.
Kaptein, E.M., Hays, M.T. and Ferguson, D.C. (1994). Thyroid hormone metabolism: a comparative evaluation. Thyroid disorders, Veterinary Clinics of North America, Small Animal Practice 24, D.C. Ferguson (ed.), W.B. Saunders Co., Philadelphia, p. 431-463.
Braverman, L.D. and Utiger, R.D. (1991). Werner and Ingbar’s The Thyroid. Lippincott, Philadelphia.
Chiovato, L., Magri, F. and Carlé, A. (2019). Hypothyroidism in context: where we’ve been and where we’re going. Advances in Therapy 36 (Suppl 2), 47–58. https://doi.org/10.1007/s12325-019-01080-8
Hauser, P., Soler, R., Brucker-Davis, F. and Weintrub, B.D. (1997). Thyroid hormones correlate with symptoms of hyperactivity but not inattention in attention deficit hyperactivity disorder. International Journal of Psychological and Neurological Endocrinology 22, 107-114. https://doi.org/10.1016/S0306-4530(96)00043-1
Jankausakas, S.S., Morelli, M.B., Gambardella, J., et al. (2021). Thyroid hormones regulate both cardiovascular and renal mechanisms underlying hypertension. Journal of Clinical Hypertension 23(2), 373-381. Open access https://doi.org/10.1111/jch.14152
O’Connor, T.M., O’Halloran, D.J. and Shanahan, F. (2000). The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. Quarterly Journal of Medicine 93, 323–333. https://doi.org/10.1093/qjmed/93.6.323
Vermiglio, F., Lo Presti, V.P. Moleti, M., et al. (2004). Attention deficit and hyperactivity disorders in offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. Journal of Clinical Endocrinology & Metabolism 89(12), 6054-6060. https://doi.org/10.1210/jc.2004-0571
Othman, S., Philips, D.I.W., Parkes, A.B., et al. (1990). A long term follow up of postpartum thyroiditis. Clinical Endocrinology 32, 559-564. https://doi.org/10.1111/j.1365-2265.1990.tb00898.x
Sharif, K., Tiosano, S. Watad, A. et al. (2018). The link between schizophrenia and hypothyroidism: a population-based study. Immunological Research 66, 663-667. https://doi.org/10.1007/s12026-018-9030-7
Soheili-Nezhad, S., Sprooten, E.. Tendolkar, I., et al. (2023). Exploring the genetic link between thyroid dysfunction and common psychiatric disorders: A specific hormonal, or a general autoimmune comorbidity. Thyroid 33(2), 159–168. Open access https://doi.org/10.1089/thy.2022.0304
Cudd, T.A., Chen, W-J. A. and West, R.W. (2002). Fetal and maternal thyroid hormone responses to ethanol exposure during the third trimester equivalent of gestation in sheep. Alcoholism: Clinical and Experimental Research 26(1), 53-58.
Roberts, C.G.P. and Ladenson, P.W. (2004). Hypothyroidism. Lancet 363, 793-803.
Hollowell, J.G., Staehling, N.W., Flanders, et al. (2002). Serum TSH, T4 and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). Journal of Clinical Endocrinology & Metabolism 87, 489-499.
Canaris, G.J., Manowitz, N.R., Mayor, G. and Ridgeway, E.C. (2000). The Colorado thyroid disease prevalence study. Archive of Internal Medicine 160, 526-534. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/415184
Singh, R.J. and Kaur, P. 2016. Thyroid hormone testing in the 21st century. Clinical Biochemistry 49(12), 843-845. Open access https://doi.org/10.1016/j.clinbiochem.2016.06.007
Arem, R. and Escalante, D. (1996). Subclinical hypothyroidism: epidemiology, diagnosis, and significance. Advances in Internal Medicine 41, 213-250.
Hanna, F.W.F., Lazarus, J.H. and Scanlon, M.F. (1999). Controversial aspects of thyroid disease. British Medical Journal 319, 894-899.
O'Reilly, D.S. (2000). Thyroid function tests—time for a reassessment. British Medical Journal 320, 1332-1334.
Stock, J.M., Surks, M.I. and Oppenheimer, J.H. (1974). Replacement dosage of L-thyroxine in hypothyroidism: a re-evaluation. New England Journal of Medicine 290(10), 529-533.
Surks, M.I., Chopra, I.J., Mariash, C.N., et al. (1990). American Thyroid Association guidelines for use of laboratory tests in thyroid disorders. Journal of the American Thyroid Association 263, 1529-1532.
Weetman, A.P. (1997). Hypothyroidism: screening and subclinical disease. British Medical Journal 314, 1175-1178. https://doi.org/10.1136/bmj.314.7088.1175
Demers, L.M. and Spencer, C.A. (eds.) (2002). Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Monograph published by the National Academy of Clinical Biochemistry, Washington, DC., see www.nacb.org
Wartofsky, L. and Dickey, R.A. (2005). The evidence for a narrower thyrotropin reference range is compelling. Journal of Clinical Endocrinology & Metabolism 90(9), 5483-5488.
Van Uytfanghe, K., Ehrenkranz, J., Halsall, D. et al. (2023). Thyroid stimulating hormone and thyroid hormones (triiodothyronine and thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status. Thyroid 33(9), 1013-1028. Open access https://doi.org/10.1089/thy.2023.0169
Fitzgerald, S.P., Falhammar, H., and Hoermann, R. (2024). Re: “Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status” by Van Uytfanghe et al. Thyroid 34(2), 274-275. https://doi.org/10.1089/thy.2023.0496
Kuś, A., Sterenborg, R.B.T.M., Haug, E.B., et al (2024). Towards personalized TSH reference ranges: A genetic and population-based approach in three independent cohorts. Thyroid 34(8), 969-979. https://doi.org/10.1089/thy.2024.0045
Staub, J. (1998). Minimal thyroid failure: effects on lipid metabolism and peripheral target tissues. European Journal of Endocrinology 138, 137-138.
Van der Spoel, E., Roelfsema, F., and van Heemst, D. 2021. Review: Within-person variation in serum thyrotropin concentrations: main sources, potential underlying biological mechanisms, and clinical implications. Frontiers in Endocrinology 12, 619568. Open access https://doi.org/10.3389/fendo.2021.619568
Andersen, S., Pedersen, K.M., Bruun, N.H. and Laurberg, P. (2002). Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. Journal of Clinical Endocrinology & Metabolism 87, 1068-1072.
Andersen, S. and Andersen, S.L. (2024). Response to Fitzgerald et al. re: “Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status.” Thyroid 34(5), 668-669. https://doi.org/10.1089/thy.2024.0049
Andersen, S., Bruun, N.H., Pedersen, K.M., et al. (2003). Biologic variation is important for interpretation of thyroid function tests. Thyroid 13(11), 1069–1078. https://doi.org/10.1089/105072503770867237
Andersen, S., Karmisholt, J., Bruun, N.H., et al. (2022). Interpretation of TSH and T4 for diagnosing minor alterations in thyroid function: A comparative analysis of two separate longitudinal cohorts. Thyroid Research 15(1), 19. Open access https://doi.org/10.1186/s13044-022-00137-1
Hansen, P.S., Brix, T.H., Sørensen, T.I.A., et al. (2004). Major genetic influence on the regulation of the pituitary-thyroid axis: a study of healthy Danish twins. Journal of Clinical Endocrinology & Metabolism 89(3), 1181-1187. https://doi.org/10.1210/jc.2003-031641
Demers and Spencer 2002:36, see above, FN #10
Kalsbeek, A., Fliers, E., Franke, A.N., et al. 2005. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 141(10), 3832-3841.
Young, J.B., Bürgi-Saville, M.E., Bürgi, U. and Landsberg, L. (2005). Sympathetic nervous system activity in rat thyroid: potential role in goitrogenesis. American Journal of Physiology: Endocrinology & Metabolism 288, E861-E867.
Greene, R.T. (1997). Thyroid testing: the full circle. Canine Practice 22, 10-11.
Ferguson, D.C. (1997). Euthyroid sick syndrome. Canine Practice 22, 49-51.
See also, for horses: Bertin, F-R., Frank, N., Breuhaus, B.A., et al. (2024). Narrative review: Diagnosis and management of thyroid disorders and thyroid hormone supplementation in adult horses and foals. Equine Veterinary Journal 56(2), 243-252. Open access https://doi.org/10.1111/evj.13981
Wise, P.M. (1999). Neuroendocrine modulation of the “menopause”: insights into the aging brain. American Journal of Physiology, Endocrinology and Metabolism 277(6), E965-E970. https://doi.org/10.1152/ajpendo.1999.277.6.E965





I wonder if the dog studies were enough to show the range of TSH difference between dogs in the coldest and dogs in the warmest environments?