Island Syndrome Part 2: Dwarfed Mammals
Thyroid phenotypes plus stress generated dwarf island mammals
[An audio version of Parts 1 and 2 are available in Episode 16 of my podcast, Bites Out Loud, here]
Island syndrome is the biological term used to describe the tendency for unique island vertebrates to possess different body size and limb proportions compared to mainland ancestors. Although a number of suggestions have been offered to explain this phenomenon of altered growth rates on islands, no formula given so far fits all situations. I contend that the individual thyroid hormone phenotype of mainland founders determines the life history traits and body size of island descendants.
[Adapted and updated from my 2006 book, Rhythms of Life, with selected references, including some updated ones]1
As I pointed out in my last essay, to the initial stress-tolerant founders that colonize island, the factors that make island habitats stressful (predator vigilance, opportunities that require decision-making, restricted movements, limited mate choice, and virtual imprisonment) may prove very stressful for individuals born in subsequent generations who by chance inherit a thyroid rhythm that is less stress-tolerant. However, on islands such stress-intolerant descendants are not free to leave.
We know that absolute levels of thyroid hormone must rise significantly during pregnancy, often to double pre-pregnancy levels (see previous essay here), because T4 is essential for fetal development and growth at all stages. When T4 levels do not rise to appropriate levels during pregnancy, premature birth or reduced uterine growth are almost inevitable, resulting in smaller than usual newborns. Because stress of any kind reduces T4 production immediately, and often profoundly, the relationship between maternal stress and fetal growth becomes paramount to understanding island syndrome.
The fallacy of food shortages
Although food limitation is often suggested as a major factor precipitating island dwarfing, there are different effects on growth precipitated by food limitations and maternal stress.2
In a pregnant female, food deprivation (fasting) primarily affects the condition of the dam, because reduced food intake reduces the amount of T3 available for her cellular and tissue functions (via reduced conversion of T4 to T3, which causes a reduction in basal metabolic rate).
Because the fetus can utilize unconverted T4, it is buffered from the direct metabolic effects of fasting unless fasting becomes prolonged. However, even short-term psychological stress caused by competition for food, or panic induced by futile searches for food (especially for females with underlying stress-intolerance), will result in profound reduction in T4 secretion from the thyroid gland—which immediately affects the growth of the developing fetus.
Therefore, I contend it is primarily the stress experienced by the dam that negatively affects the developing fetus, not the reduced maternal basal metabolic rate resulting from the food shortage itself.
Restricted fetal growth and island dwarfs
It has been shown that small offspring produced because of reduced fetal growth seldom recoup this loss: even if growth is not restricted afterward, premature and low-birth-weight individuals remain small throughout life. And since reduced fetal growth rates appear to be inherited (passed on to offspring), small stature resulting from maternal stress can have population-level impacts over time.3
It has also been demonstrated that small individuals mature earlier on average than larger ones, and for late-maturing large animals, such as elephants, this disparity can amount to several years difference. For example, a small female elephant that matures at eleven years may be capable of producing several more offspring over her lifetime than a female that matures at fifteen years—and so will her small, early-maturing daughters.4
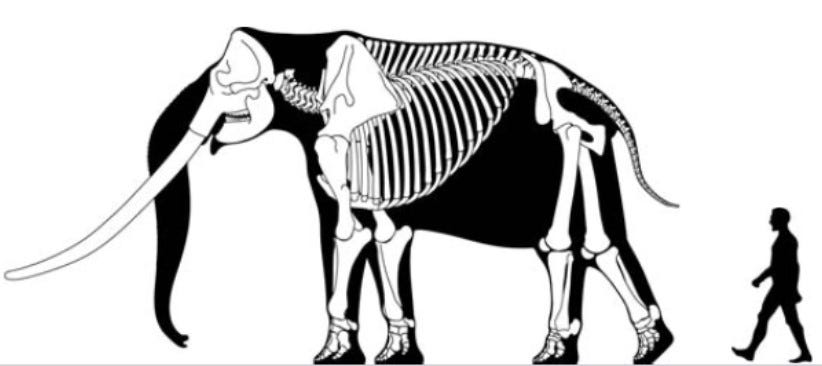
An extreme example of this phenomenon is seen in the profoundly dwarfed straight-tusked elephant that lived on Sicily during the Pleistocene (about one million years ago): these one-metre-tall elephants appear to have reached sexual maturity as early as three to four years of age.
[In smaller animals, especially those that first breed at one year of age or less, early maturation may be very subtle, as we saw in the experimental population of Siberian silver foxes discussed in a previous essay here: in those silver foxes, the earliest breeding females every season were ahead of the rest by only a few weeks but they were always stress-tolerant (“less fearful”) individuals. Smallish, early-breeding animals may well be the most stress-tolerant individuals within many large-bodied mammalian populations.]
Deliberate colonization and domestication bring such early maturing individuals together within founding populations because only stress-tolerant individuals participate in these events. And because the only growth programs available among these founders are the specific thyroid rhythm patterns that produce stress-tolerant early breeders, most offspring will inherit similar stress-tolerance (along with other behavioural and morphological traits associated with those specific growth programs).
However, a few offspring of these original colonizers will invariably have somewhat lower stress-tolerance than their founder parents, a phenomenon that may recur in subsequent generations. Under circumstances of domestication and mainland colonization, individuals with reduced stress-tolerance are free to leave the stressful new habitat and return to the original population; in island colonization, such freedom to emigrate doesn’t exist.
The lack of freedom to escape becomes a constant stressor for these stress intolerant individuals and this will have particularly strong effects on pregnant females.
Pregnant females of large animals born during the early generations of island colonization which have reduced stress-tolerance are likely to produce smaller, earlier maturing offspring. This is a direct result of the unique stresses involved in living on an island in conjunction with the effects of maternal stress on fetal growth.
In small founding populations especially, smaller, earlier maturing individuals will quickly outnumber large individuals simply because of their faster generation times, not because of food shortages. Large individuals don’t disappear entirely, they just become rare: indeed, as already mentioned, one of the characteristics of island populations is a pronounced variation in size and morphology relative to mainland forms. For example, the population of extinct mammoths on Santa Rosa Island off California has been estimated to have had one large mammoth for every ten dwarfed ones.5
Reduced food and fetal growth
In other words, shortage of food due to rapid population growth (habitat saturation) is unlikely to be a driving force in the early stages of island colonization: on the contrary, unless an island is extremely small there should be enough resources for many generations before the equilibrium point between population size and available food is surpassed.
However, this is not to say that food cannot be depleted past a critical point: once the equilibrium point between number of animals and available food is surpassed, different levels of nutritional stress can have a similar impact on a population as the initial stresses of colonization because animals must compete for food.
As discussed above, pregnant females under stress due to competition for food will produce smaller offspring which subsequently produce small offspring themselves. Thus, a trend toward small size may also take place among island populations if conditions of prolonged competition for food exists, as has been proposed for Spitsbergen reindeer.
To summarize, then: in domestication, stress-tolerant individuals of large mammals are interbreeding exclusively within a small founder population where escape—emigration—is possible, and these produce slightly dwarfed offspring due to the reduced variation in growth programs available among founders (emigration of any stress-intolerant individuals born in early generations keep the effects from being extreme).
In contrast, in island colonization, stress-intolerant individuals born in the early generations produce much more profoundly dwarfed offspring because the environment is emigration-proof; if island founders include a few stress-intolerant individuals from the very beginning, as may happen when once-connected islands populated during times of lower sea levels suddenly become cut off by rapidly rising seas, even more profound dwarfing can occur.
Both moderate and extreme dwarfing can occur on the same island at different times due to repeated colonization events by the same ancestral species (as happened, for example, on the island of Sicily during the Pleistocene: here the extremely dwarfed elephant came first and the only moderately dwarfed form came later—although both are now extinct).
Remains of extremely dwarfed individuals have been recorded on islands within 4,000 to 6,000 years after initial colonization events in extinct large mammals (such as red deer on the Isle of Jersey in the English Channel and mammoths on Wrangel Island on the northeast coast of Siberia), although the process almost certainly begins soon after arrival and proceeds rapidly.6
Stegodon dwarfing in Indonesia
Here’s one example from Indonesia of what can happen with island colonization.
The generalities outlined above can be used to explain the fossil record of the primitive Southeast Asian elephants known as stegodons on Flores Island, one of the larger islands of the Lesser Sunda archipelago that lies east of Java. Flores was never connected to the islands on either side, but was isolated by deep channels that narrowed just enough (to ca. 25 km) during times of extremely low sea levels to allow deliberate colonization by a full-sized Stegodon from Java (via Bali and Lombok to the west).
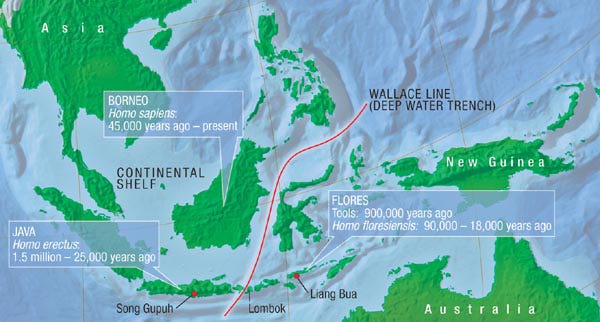
Colonization of Flores by stegodons from Java occurred at least twice: once at about 1,500,000 years ago (ya) and again at about 800,000 ya. The first colonization resulted in the generation of Stegodon sondaari, the smallest of all Indonesian stegodon species.
This dwarf stegodon was tiny: it had an estimated weight of about 300 kg compared to 1,000–1,700 kg for the ancestral form (much less than half its size) and its molar teeth had more complex cusp patterns than its ancestor. The most recent well-documented remains of this dwarfed form date to about 900,000 ya but the species appears to have become extinct shortly after, suggesting it survived on Flores for about half a million years.7
At about 800,000 ya, a second Stegodon colonization of Flores occurred, associated with yet another major drop in sea level. This group of stegodon colonizers evolved into Stegodon florensis, which survived somewhat longer than the previous species. However, Stegodon florensis is estimated to have weighed about 850 kg and so was only moderately dwarfed compared to mainland forms (about half its size or larger). Stegodon florensis is described as the most common fossil of Pleistocene Flores, suggesting that a thriving population of this species existed before it became extinct sometime before 12,000 ya in the wake of a major volcanic eruption.8
The critical question appears to be why the first wave of stegodons became extremely dwarfed but the second did not, given similar lengths of occupation of the same island.
[Note that reports that the very late Pleistocene stegodons on Flores Island were also extremely dwarfed (as described by Mike Morwood and colleagues in 2004), are apparently an error.]9
As I mentioned briefly above, most examples of extremely dwarfed large mammals are associated with islands that were once connected to other land masses, thus presenting an opportunity for a small colony of both stress-tolerant and stress-intolerant individuals (a hormonally heterogeneous mix) to become stranded together.
This pattern suggests that if early Flores stegodons were extremely dwarfed, they should be descendants of a hormonally heterogeneous founding population. But how could a hormonally heterogeneous group of stegodons get to Flores, when it was never connected to other islands in the archipelago?
Turned around this way, perhaps the really significant question is not why Late Pleistocene stegodons on Flores did not become extremely dwarfed but why the earlier population did. At least two possible scenarios can be suggested.
The first possibility is that the ancestral populations were different: while both early and middle Pleistocene ancestors technically belonged to the same species of Stegodon, they may not have been precisely equivalent. Colonizing founders from the two time periods may have differed enough in degree of stress tolerance to generate distinct descendants.
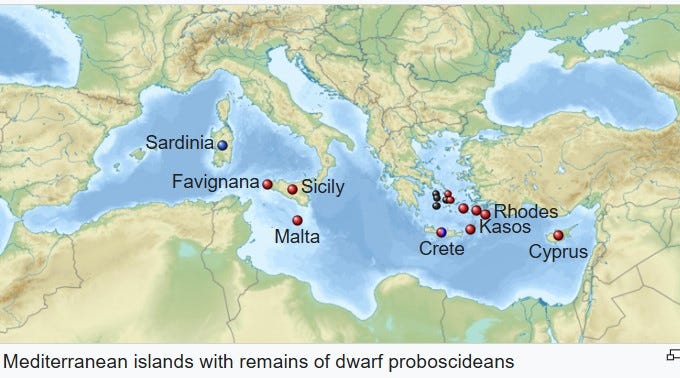
This is precisely what has been suggested by researchers trying to assign appropriate scientific names to the many populations of elephants that inhabited islands of the Mediterranean during the Pleistocene, which all descended from a mainland straight-tusked elephant, Elephas antiquus.10
There is another, perhaps equally likely explanation for extreme dwarfing in the earlier Flores population, and that is that the extended social bonds of elephant mothers and offspring encouraged a more physiologically and behaviourally heterogeneous mix of stegodons to swim offshore than occurs in other deliberately-colonizing large mammals.
The prolonged mother/calf bond characteristic of elephants and their Pleistocene relatives especially, and perhaps to a lesser degree in Pleistocene hippos (as for modern Hippopotamus amphibious), might induce stress-intolerant offspring – even as adults – to accompany stress-tolerant mothers on an offshore swim they would not undertake alone.
This would explain why primitive elephants and their relatives, as well as hippos, often (but not always) became extremely dwarfed even when deliberate colonization events were involved.11
The second wave of stegodon colonizers may have been only moderately dwarfed because on this occasion the population derived from a deliberate colonization event by a few stress-tolerant individuals only, generating less-extreme changes in growth programs.
However, the possibility also exists that the second colonization event was different from the first because low sea levels during that period of the Pleistocene brought a new predator to Flores: Homo erectus.
A viable population of near normal-sized stegodons may have survived longer than the previous extremely dwarfed population because hunting by Homo erectus kept the animals relatively large and late-maturing. Hunting would also have kept the population small enough to prevent habitat saturation and subsequent dwarfing due to food shortages.
As stated previously, a similar pattern of early colonizers becoming extremely dwarfed while later ones did not is also documented for the Pleistocene straight-tusked elephants that colonized the Mediterranean island of Sicily: remains of large carnivores are found on Sicily in association with fossils of medium-sized dwarfed forms, suggesting predation on small individuals of that elephant population may have occurred.
Evidence does indeed exist for late Pleistocene predation of stegodons on Flores: age profiles done on associated Stegodon remains suggest that juveniles were preferentially targeted by late Pleistocene hunters, although in earlier times very small adults (if they existed) may also have been taken.
My next essay will focus on that other Flores Island dwarf, Homo floresiensis, the hominin species descended from Homo erectus reported in 2004 and nicknamed The Hobbit.
The easiest thing you can do to support Biology Bites is to click the “♡ Like”. The more likes = the higher this post rises in the Substack feeds, which puts Biology Bites in front of more readers.
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria.
Carlquist, S. (1965). Island Life: A Natural History of the Islands of the World. The Natural History Press, Garden City.
Sondaar, P.Y. (1977). Insularity and its effect on mammal evolution. In M.K. Hecht, P.C. Goody and B.M. Hecht (eds), Major Patterns in Vertebrate Evolution. New York: Plenum. p 671-707.
Forchhammer M.C. (2000). Timing of foetal growth spurts can explain sex ratio variation in polygynous mammals. Ecology Letters 3, 1-4.
Palkovacs E.P. (2003). Explaining adaptive shifts in body size on islands: a life history approach. Oikos 103, 37-44. https://doi.org/10.1034/j.1600-0706.2003.12502.x Request pdf here.
Caloi, L., Kotsakis, T., Palombo, M.R. and Petronio, C. (1996). The Pleistocene dwarf elephants of Mediterranean islands. In J. Shoshani and P. Tassy (eds), The Proboscidea: The Evolution and Palaeoecology of Elephants and their Relatives, pg. 234-239. Oxford: Oxford University Press.
Lister, A.M. (1989). Rapid dwarfing of red deer on Jersey in the last Interglacial. Nature 342, 539-542.
Lister, AM. (2004). The impact of Quaternary Ice Ages on mammalian evolution. Philosophical Transactions of the Royal Society of London B. 359, 221-241.
Vartanyan, S.L., Garutt, V.E. and Sher, A.V. (1993). Holocene dwarf mammoths from Wrangel Island in the Siberian Arctic. Nature 362, 337-340.
van den Bergh, G.D. (1999). The Late Neogene elephantoid-bearing faunas of Indonesia and their paleozoogeographic implications. Scripta Geologica 117.
Morwood M.J., R.P. Soejono, R.G. Roberts, T. et al. (2004). Archaeology and age of a new hominin from Flores in eastern Indonesia. Nature 431, 1087-1091.
J. de Vos, personal communication to S.J. Crockford, 2005.
Palombo, M.R. and Ferretti, M.P. (2005). Elephant fossil record from Italy: knowledge, problems and perspectives. Quaternary International 126, 107-136.
Hanken, J. and Wake, D.B. (1993). Miniaturization of body size: Organismal consequences and evolutionary significance. Annual Review of Ecology and Systematics 24, 501-519.