Testing the Thyroid Rhythm Hypothesis
My thyroid hormone theory for how speciation works is scientific because it's testable: here's what needs to be done
[Adapted from my 2006 book, Rhythms of Life, with selected references, including some updated ones]1
Ultimately, the reason my theory that thyroid hormones play a critical role in evolution—including human evolution—is so important, is because my hypotheses are testable.
Being testable is what makes this theory scientific and distinguishes it from other wishful-thinking narratives and “just-so” stories of how species arise.2
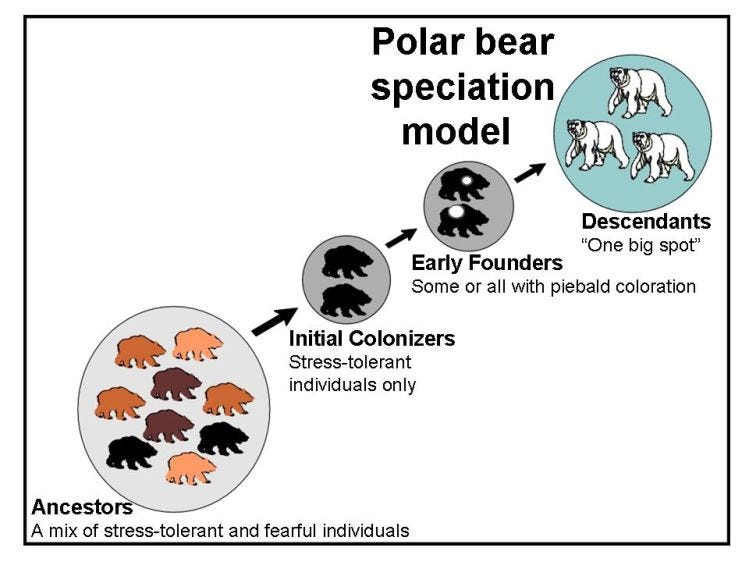
Thyroid rhythm theory assumes that individually-unique thyroid rhythm variants exist within species-specific patterns for animal populations and that these thyroid rhythm variants are the actual characteristics targeted by natural selection in instances of adaptation and colonization.
The model predicts that non-random subdivision of populations often occurs during speciation, isolating particular subsets of individuals with similar thyroid rhythms within founder populations. Developmental repercussion of reduced thyroid rhythm diversity in the founding group is assumed to be responsible for generating the particular growth and development changes seen in descendant taxa.
The basic hypothesis to be tested, therefore, is that daily rhythmic thyroid hormone (TH) secretion profiles in any vertebrate species are individually variable and that these variations between individuals can be correlated with discernable physical, reproductive and behavioural differences, generating thyroid rhythm phenotypes.
As a group, individual thyroid rhythm phenotypes together should generate a distinctive pattern for the population that is species-specific (or in the case of domesticates, breed-specific); that is, the average pattern for the group should be distinguishable from that of a closely-related species.
Devising experiments that can reliably test this premise will undoubtedly be difficult due to the dynamic nature of the endocrine system and its inherent sensitivity to stress of any kind. Such sensitivity presents a unique challenge to the determination of normal thyroid rhythms within and between taxa, since TH levels must be measured frequently (at least every 5–7 minutes, perhaps as often as 2.5 minutes), under controlled conditions and for many individuals.
Experimental tests have demonstrated that automated sampling may circumvent many of the difficulties of testing the thyroid rhythm hypothesis.
For one study, which measured levels of the adrenal hormone corticosterone in rats, a surgically implanted cannula connected to an automated blood-sampling apparatus allowed minute quantities of blood (10–20 μl) to be collected every ten minutes over a twenty-four-hour period without disturbing the animals by repeated handling.3
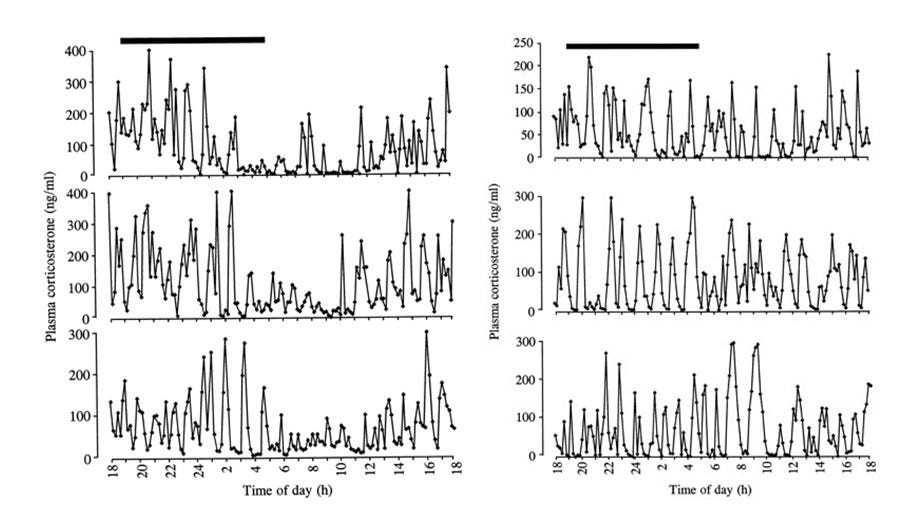
The two breeds of rats used in this study had significant differences in mean profiles of hormone production (as well as slight individual variations within breeds) in addition to significant differences in behavioural responses to a controllable stress (so-called white noise). The success of these experiments in demonstrating the existence of fine-scale patterns of corticosterone production and in correlating these profiles to stress responses suggests that a similar method might be suitable for testing the thyroid rhythm hypothesis.
Similar automated sampling methods have also been applied to studies on pulsatile hormone secretion in humans and a range of other species—including cats, rats, horses, cattle, chickens, frogs and fish. In my opinion, such active areas of research simply need an over-arching paradigm to put the results into evolutionary context.4
While huge advances have been made in measuring cortisol, the adrenal stress-hormone analogue to corticosterone found in fish, humans, and other medium to large sized mammals, doing something similar for TH presents unique problems.5
That’s because current laboratory assay methods for measuring T3 or T4 in minute quantities (as opposed to measuring TSH - thyroid stimulating hormone or thyrotropin) may place limitations on the smallest samples that can be analyzed. So unless it can be demonstrated that using TSH is superior for this particular purpose, it means new assay methods may need to be developed.6
The sampling apparatus itself may need modification to allow testing of a full range of animals: laboratory-housed fish species and free-ranging bears, for example, pose very different logistical problems for an automated TH sampling device.
However, the advances in similar devices have been made over the last two decades suggest that success with TH sampling tools are within reach.
If individual variation within species-specific profiles of thyroid rhythms can be confirmed, controlled breeding experiments – similar to those described for silver foxes – will be necessary to confirm that small interbreeding groups of animals with similar thyroid rhythm profiles produce descendants of a different type within twenty generations or less.
[Although a few experiments of this nature have been done, so far they lack the necessary sampling of incremental TH levels.]7
It would be most convincing if descendants of such breeding programs could have their thyroid rhythm profiles monitored as well, for these should differ from the original source population.
If it can be demonstrated that thyroid rhythms are indeed variable within certain limits for different populations (or breeds, or for certain morphs within species) and that heterochronic changes can be generated by interbreeding small groups of physiologically similar individuals, the final step will be to find the genetic sources of those pattern differences.
Although species-specific thyroid rhythms are probably controlled by genes in the suprachiasmatic nucleus (SCN) of the hypothalamus that are directly associated with generating hormone pulsatility, other factors may affect thyroid hormone utilization in ways that are also species-specific, such as the different concentrations of the thyroid hormone-transporting molecule transthyretin found in chimpanzees and humans by one research group.8
Mutations in genes controlling other aspects of thyroid-mediated actions in target tissues (such as receptors, receptor ligands and/or cofactors) are also potential causes of variation.
However, it is expected that in most cases, differences in such genes will be found to supplement, compound, or confound thyroid rhythm effects, rather than contribute to their initial rhythmic generation – and as a consequence, genes involved in such thyroid hormone-mediated “end-factor” processes could be selected for independently from thyroid rhythms or simultaneously. This means that mutations in such genes may explain the origins of some evolutionary novelties that are not developmental in nature.
I suggest that the first step in genetic characterization of individual and species-specific thyroid rhythm profiles should start with documenting variation in SCN output.
In the meantime, ongoing research into the regulatory mechanisms of embryonic development should unravel some of the essential molecular interactions that involve thyroid hormones and thyroid rhythms.
Research on Hox genes that act during embryonic development has thus far revealed they respond to retinoic acid as well as to other molecules. In light of the known developmental regulation functions that retinoic acid shares with thyroid hormone, or in which their roles cannot be distinguished, it would be prudent for researchers to look at the response of Hox genes to TH and thyroid rhythm pulses, in combination with retinoic acid.9
Given the critical roles recently demonstrated for thyroid hormones themselves in embryonic development, we also need to know what effects different thyroid rhythm profiles might have on any given developmental program.
Lastly, research into the physiological and genetic basis of natural piebaldness (rather than aberrant whitespotting mutants) may also be illuminating. Piebaldness, if we can come to understand exactly what it signifies in an evolutionary context, could serve as an especially useful diagnostic marker for developmental change.
As I’ve said before:
While this paradigm challenges one of the accepted tenets of neo-Darwinian theory ─ that evolution is due almost exclusively to the gradual accumulation of random genetic mutations and as a consequence is almost always imperceptibly slow ─ it more accurately reflects and predicts the complex nature of inter- and intra-specific relationships we are now able to discern from phylogenetic analysis of closely related species, including our own ancestors. Significantly, it can explain the big, body-plan changes associated with macroevolution that are often seen as problematic.
My hypothesis offers an explanation that plugs many of the gaps in our understanding of how and why speciation works. If it is not this mechanism exactly, it must be one like it.
The only way to find out if the mechanism I’ve described is entirely correct, or only partially so, is to undertake the kinds of tests described above, which are too multi-faceted to fall on one person (me). My job was to formulate the paradigm and lay out its foundation and the associated hypotheses, which I did in my 2004 dissertation: the actual work of testing it falls to a new generation of evolutionary biologists looking to make their mark.
Crockford, S.J. (2006). Rhythms of Life: Thyroid Hormone and the Origin of Species. Trafford, Victoria.
See also: Crockford, S.J. (2004). Animal Domestication and Vertebrate Speciation: A Paradigm for the Origin of Species. Ph.D. dissertation. University of Victoria, Canada. http://hdl.handle.net/1828/542
Horrobin, D. (2001). The Madness of Adam and Eve: How Schizophrenia Shaped Humanity. Bantam Press, London. [I met Dr. Horrobin before he died and we talked extensively about speciation mechanisms: he agreed that my hypothesis was was testable as an evolutionary theory and his was not]
Wheeler, P.E. (1984). The evolution of bipedality and loss of functional body hair in humans. Journal of Human Evolution 13, 91-98. https://doi.org/10.1016/S0047-2484(84)80079-2
Lightman, S.L., Windle, R.J., Julian, M.D., et al. (2000). Significance of pulsatility in the HPA axis. Mechanisms and Biological Significance of Pulsatile Hormone Secretion, D.J. Chadwick and J.A. Goode (eds.), J. Wiley and Sons, Chichester, p. 244-260.
Windle, R.J., Wood, S.A., Lightman, S.L. and Ingram, C.D. (1998a). The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology 139(10), 4044-4052. https://doi.org/10.1210/endo.139.10.6238
Windle, R.J., Wood, S.A., Shanks, N., (1998b). Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139(2), 443-450. https://doi.org/10.1210/endo.139.2.5721
Adcock, C.J., Ogily-Stuart, A.L., Robinson, I.C., et al. (1997). The use of an automated microsampling system for the characterization of growth hormone pulsatility in newborn babies. Pediatric Research 42(1), 66-71. Open access https://doi.org/10.1203/00006450-199707000-00011
Lucke, C., Hehrmann, R., von Mayersbach, K. and von zur Muhlen, A. (1977). Studies on circadian variations of plasma TSH, thyroxine and triiodothyronine in man. Acta Endocrinologica 86(1), 81-88. https://doi.org/10.1530/acta.0.0860081
Bitman, J., Kahl, S., Wood, D.L. and Lefcourt, A.M. (1994). Circadian and ultradian rhythms of plasma thyroid hormone concentrations in lactating dairy cows. American Journal of Regulatory, Integrative and Comparative Physiology 266, 1797-1803.
Campos-Barros, A., Musa, A., Flechner, A., et al. (1997). Evidence for circadian variations of of thyroid hormone concentrations and type II 5’-iodothyronine deiodinase activity in the rat central nervous system. Journal of Neurochemistry 68, 795-803.
Cogburn, L.A. and Freeman, R.M. (1987). Response surface of daily thyroid hormone rhythms in young chickens exposed to constant ambient temperature. General and Comparative Endocrinology 68(1), 113-123.
Gomez, J.M., Boujard, T., Boeuf, G., et al., (1997). Individual diurnal profiles of thyroid hormones in rainbow trout (Oncorhynchus myskiss) in relation to cortisol, growth hormone, and growth rate. General and Comparative Endocrinology 107(1), 74-83. https://doi.org/10.1006/gcen.1997.6897
Gupta, B.B.P. and Premabati, Y. (2002). Differential effects of melatonin on plasma levels of thyroxine and triiodothyronine levels in the air-breathing fish, Clarias gariepinus, during breeding and quiescent periods. General and Comparative Endocrinology 129, 146-151. https://doi.org/10.1016/S0016-6480(02)00527-0
Duckett, W.M., Manning, J.P. and Weston, P.G. (1989). Thyroid hormone periodicity in healthy adult geldings. Equine Veterinary Journal 21(2), 123-125. https://doi.org/10.1111/j.2042-3306.1989.tb02115.x
Gancedo, B., Alonso-Gomez, A.L., de Pedro, N., et al. (1997). Changes in thyroid hormone concentrations and total contents through ontogeny in three anuran species: evidence for daily cycles. General and Comparative Endocrinology 107, 240–250. https://doi.org/10.1006/gcen.1997.6922
Wright, M.L., Duffy, J.L., Guertin, C.J., et al. (2003). Developmental and diel changes in plasma thyroxine and plasma and ocular melatonin in the larval and juvenile bullfrog, Rana catesbeiana. General and Comparative Endocrinology 130, 120-128. https://doi.org/10.1016/S0016-6480(02)00575-0
Vignesh, V., Castro-Dominguez, B., James, T.D., et al. (2024). Advancements in cortisol detection: from conventional methods to next-generation technologies for enhanced hormone monitoring. ACS Sensors 9(4), 1666-1681. Open access https://doi.org/10.1021/acssensors.3c01912
Andersen, S., Karmisholt, J., Bruun, N.H., et al. (2022). Interpretation of TSH and T4 for diagnosing minor alterations in thyroid function: A comparative analysis of two separate longitudinal cohorts. Thyroid Research 15(1), 19. Open access https://doi.org/10.1186/s13044-022-00137-1
Leirs, K., Dosso, F.D., Perez-Ruiz, E. et al. (2022). Bridging the gap between digital assays and point-of-care testing: automated, low cost, and ultrasensitive detection of thyroid stimulating hormone. Analytical Chemistry 94(25), 8919-8927. https://doi.org/10.1021/acs.analchem.2c00480
Van Uytfanghe, K., Ehrenkranz, J., Halsall, D. et al. (2023). Thyroid stimulating hormone and thyroid hormones (triiodothyronine and thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status. Thyroid 33(9), 1013-1028. Open access https://doi.org/10.1089/thy.2023.0169
Van der Spoel, E., Roelfsema, F., and van Heemst, D. (2021). Review: Within-person variation in serum thyrotropin concentrations: main sources, potential underlying biological mechanisms, and clinical implications. Frontiers in Endocrinology 12, 619568. Open access https://doi.org/10.3389/fendo.2021.619568
Esposito, A., Ambrosino, L., Piazza, S., et al. (2021). Evolutionary adaption of the thyroid hormone signaling toolkit in chordates. Cells 10, 3391. https://doi.org/10.3390/cells10123391
Esin, E.V., Shulgina, E.V., and Shkil, F.N. (2023). Rapid hyperthyroidism-induced adaptation of salmonid fish in response to environmental pollution. Journal of Evolutionary Biology 36 (10), 1471–1483. Open access. https://doi.org/10.1111/jeb.14220
Roux, N., Miura, S., Dussene, M., et al. (2022). The multi-level regulation of clownfish metamorphosis by thyroid hormones. bioRxiv https://doi.org/10.1101/2022.03.04.482938
Salis, P., Roux, N., Huang, D., et al. (2021). Thyroid hormones regulate the formation and environmental plasticity of white bars in clownfishes. Proceedings of the National Academy of Sciences USA 118, e2101634118. https://doi.org/10.1073/pnas.2101634118
Smirnov, S.V., Kapitanova, D.V., Borisov, V.B., et al. (2012). Lake Tana large barbs diversity: Developmental and hormonal bases. Journal of Ichthyology 52, 861–880. https://doi.org/10.1134/S0032945212110082
Shkil, F.N. and Smirnov, S.V. (2015). Experimental approach to the hypothesis of heterochronic evolution in lower vertebrates. Paleontological Journal 49, 1624–1634. https://doi.org/10.1134/S0031030115140178
Woronowicz, K.C., Esin, E.V., Markevich, G.N., et al. (2023). Phylogenomic analysis of the Lake Kronotskoe species flock of Dolly Varden charr reveals genetic signatures of sympatric radiation. bioRxiv 2023.02.24.529919. https://doi.org/10.1101/2023.02.24.529919
Gagneux, P., Amess, B. Diaz, S., et al. (2001). Proteomic comparison of human and great ape blood plasma reveals conserved glycosylation and differences in thyroid hormone metabolism. American Journal of Physical Anthropology 115, 99-109.
See also Beringer, V. Deschner, T., Murtagh, R. et al. (2014). Age-related changes in thyroid hormone levels of bonobos and chimpanzees indicate heterochrony in development. Journal of Human Evolution 66, 83–88. https://doi.org/10.1016/j.jhevol.2013.09.008
Lawrence, P.A. and Morata, G. (1994). Homeobox genes: their function in Drosophila segmentation and pattern formation. Cell 78, 181-189. https://doi.org/10.1016/0092-8674(94)90289-5
Barres, B.A., Lazar, M.A. and Raff, M.C. (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097-1108. Open access https://doi.org/10.1242/dev.120.5.1097
Evans, R.M. (1988). The steroid and thyroid hormone receptor superfamily. Science 240, 889-895.
Stephanou, A. and Handwerger, S. (1995). Retinoic acid and thyroid hormone regulate placental lactogen expression in human trophoblast cells. Endocrinology 136, 933-938.